Introduction and Safety
15
26
1
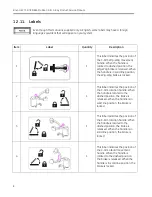
Unique Device Identifier (UDI)
label(US only)
Every medical device has a unique
marking for identification.
Note that this is only an example of a
UDI marking.
The characters used in the UDI
marking represent specific
identifiers.
Device identifier:
(01)= GS1 global trade item
number (GTIN) of the device.
00001234567895 = Global trade
item number.
Production identifiers:
(11) = GS1 application identifier
for the manufacturing date of
the device.
150700= Manufacturing date:
year-month-day (YYMMDD) with
DD always equal to 00.
(21) = GS1 application identifier
for the serial number of the
device.
AAAAAYY00001AA = Serial
number.
27
1
This label indicates the system
was tested by ITS authorized
body and was found to be in
compliance with the
requirements of all relevant
directives and standards at the
time of manufacture.
28
1
This label can be found on the X-
Ray generator. It indicates the
location of the X-Ray source and
the controls used to produce
ionizing X-Radiation. Use
appropriate precautions and
protective equipment when using
the system and at all time when
X-Rays are present.
Summary of Contents for Brivo OEC 715
Page 2: ......
Page 19: ...Chapter1 Introduction and Safety...
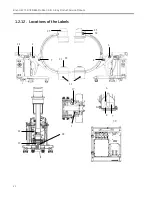
Page 41: ...Introduction and Safety 23 46 54 20 18 26...
Page 55: ...Chapter2 System Overview...
Page 137: ...Chapter3 Installation...
Page 212: ...Chapter4 Calibration...
Page 275: ...Brivo OEC 715 785 865 Mobile C Arm X Ray Product Service Manual 4 64...
Page 284: ...Chapter5 Software...
Page 326: ...Software 5 43 2 Click on install to continue 3 Click Next to continue...
Page 335: ...Chapter6 Troubleshooting...
Page 408: ...Chapter7 Replacement...
Page 418: ...Replacement 7 11 166...
Page 488: ...Chapter8 Periodic Maintenance...
Page 502: ...Periodic Maintenance 8 15...
Page 505: ...Chapter9 Technical Reference...
Page 521: ...Technical Reference 9 17 Vertical configuration 1 5m Vertical configuration 1m...
Page 526: ...11 Appendix System Schematics...