GE M
EDICAL
S
YSTEMS
D
IRECTION
2380207, R
EVISION
7
LOGIQ™ 5 PRO S
ERVICE
M
ANUAL
1-16
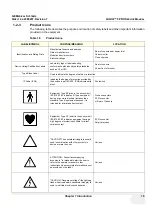
Section 1-3 - Safety Considerations
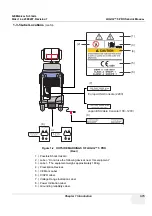
1.) UL Label
2.) Identification and Rating Plate - USA/Asia 120V Console
3.) Identification and Rating Plate - Europe/Asia/Latin America 220V Console
4.) Identification and Rating Plate - Japan 100V Console
5.) Identification and Rating Plate - Korea 220V Console
6.) Caution EIAJ Label
7.) Identification and Rating Plate - China 220V Console
Figure 1-3 OUTSIDE MARKINGS OF LOGIQ™ 5 PRO
Korea 220V Console
(1)
(2)
(3)
(4)
(5)
(6)
GE ULTRASOUND KOREA LTD.
1250VA
1250VA
1250VA
120V
GE ULTRASOUND Korea
GE ULTRASOUND KOREA
China 220V Console
(7)
LOGIQ 5 PRO
LOGIQ 5 PRO
LOGIQ 5 PRO
LOGIQ 5 PRO
LOGIQ 5 PRO
LOGIQ 5 PRO
Содержание LOGIQ 5 PRO
Страница 2: ...GE MEDICAL SYSTEMS DIRECTION 2407381 REVISION 7 LOGIQ 5 PRO SERVICE MANUAL Page 1 2 ...
Страница 3: ......
Страница 7: ...GE MEDICAL SYSTEMS DIRECTION 2380207 REVISION 7 LOGIQ 5 PRO SERVICE MANUAL ii iii ...
Страница 50: ...GE MEDICAL SYSTEMS DIRECTION 2380207 REVISION 7 LOGIQ 5 PRO SERVICE MANUAL 2 12 Section 2 3 Facility Needs ...
Страница 462: ...GE MEDICAL SYSTEMS DIRECTION 2380207 REVISION 7 LOGIQ 5 PRO SERVICE MANUAL A 4 Index INDEX ...