CHAP
. 13
GE Healthcare
Senographe 2000 D Acquisition System
REV 1
OM 5179217–1–100
140
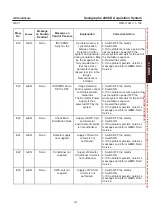
Streptococcus faecalis, ATCC 19433
Streptococcus pyogenes, ATCC 19615
Trichophyton mentagrophytes
Mycobacterium tuberculosis var. bovis
Influenza A
2
Japan
Herpes simplex type 2
vaccinia
adenovirus type 2
HIV–1 (AIDS) virus
LpHse
, manufactured by Calgon Vestal Laboratories, St. Louis, MO, U.S.A., EPA Reg. No. 1043–92
(510(k) K931342)
According to the manufacturer, when used according to the manufacturer’s instructions, this product is
effective against all of the above, with the exception of Salmonella choleraesuis.
1-6-2
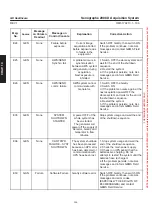
High Level Disinfection
CIDEX
, manufactured by Johnson & Johnson Medical, Inc., Arlington, TX, U.S.A., EPA Reg. No.
7078–1, EPA Est. No. 36126–PR–1 (510(k) K924434
CIDEX (A CLEANING SOLUTION) CONTAINS GLUTARALDEHYDE. DIRECT
CONTACT IS CORROSIVE TO EXPOSED TISSUE, CAUSING EYE DAMAGE AND
SKIN IRRITATION/DAMAGE. DO NOT GET INTO EYES, ON SKIN OR ON
CLOTHING. USE IN WELL VENTILATED AREA IN CLOSED CONTAINERS.
DANGER
FOR
TRAINING
PURPOSES
ONLY!
NOTE:
Once
downloaded,
this
document
is
UNCONTROLLED,
and
therefore
may
not
be
the
latest
revision.
Always
confirm
revision
status
against
a
validated
source
(ie
CDL).