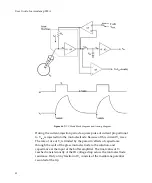
Guide
to
Electrophysiological
Recording
Filling
Electrodes
Only
the
taper
and
a
few
millimeters
of
the
shaft
of
the
pipette
should
be
filled
with
solution.
The
chlorided
tip
of
the
wire
should
be
inserted
into
this
solution.
Avoid
wetting
the
holder
since
this
will
increase
the
noise.
Silver
Chloriding
It
is
up
to
you
to
chloride
the
end
of
this
wire
as
required.
Chloriding
procedures
are
contained
in
many
electrophysiology
texts.
For
easy
‐
to
‐
use
recipes
see
Microelectrode
Methods
for
Intracellular
Recording
and
Iontophoresis
by
Purves
(page
51)
and
The
Axon
Guide
(page
83).
Typically
the
chlorided
wire
will
need
to
be
replaced
or
rechlorided
every
few
weeks.
A
simple
yet
effective
chloriding
procedure
is
to
clean
the
silver
wire
down
to
the
bare
metal
using
fine
sand
paper
and
immerse
the
cleaned
wire
in
bleach
for
about
20
minutes,
until
the
wire
is
uniformly
blackened.
This
provides
a
sufficient
coat
of
AgCl
to
work
reliably
for
several
weeks.
Drifting
or
otherwise
unstable
offsets
during
experiments
is
suggestive
of
the
need
for
rechloriding.
The
chlorided
region
should
be
long
enough
so
that
the
electrode
solution
does
not
come
in
contact
with
the
bare
silver
wire.
Heat
smoothing
the
back
end
of
the
electrode
extends
the
life
of
the
chloride
coating
by
minimizing
the
amount
of
scratch
damage.
Another
way
to
protect
the
AgCl
coating
is
to
slip
a
perforated
Teflon
tube
over
the
chlorided
region.
Holder
Maintenance
Cleaning
For
lowest
noise,
keep
the
holder
clean.
Frequently
rinse
the
holder
with
distilled
water.
If
more
thorough
cleaning
is
required,
briefly
wash
in
ethanol
or
mild
soapy
water.
Never
use
methanol
or
strong
solvents.
81