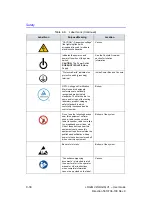
Safety Precautions
LOGIQ V2/LOGIQ V1
–
User Guide
4-31
Direction 5610736-100
Rev. 9
Name and Concentration of Hazardous Substances
Table 4-5: Table of hazardous substances' name and concentration for LOGIQ V2/
LOGIQ V1
Component Name
Hazardous substances' name
Pb
Hg
Cd
Cr (VI)
PBB
PBDE
LCD Panel
X
O
O
O
O
O
Printed Circuit Board
Assemblies
X
O
O
O
O
O
Keyboard Assemblies
X
O
O
O
O
O
Power Assemblies
X
O
O
O
O
O
Battery
X
O
O
O
O
O
Ultrasound Probes
X
O
O
O
O
O
This table is prepared according to SJ/T 11364.
O: Indicates that hazardous substance contained in all of the homogeneous materials for this part is below
the limit requirement in GB/T 26572.
X: Indicates that hazardous substance contained in at least one of the homogeneous materials used for
this part is above the limit requirement in GB/T 26572.
• Data listed in the table represents best information available at the time of publication.
• Applications of hazardous substances in this medical device are required to achieve its intended clinical
uses, and/or to provide better protection to human beings and/or to environment, due to lack of reasonably
(economically or technically) available substitutes. For example: Lead is could be used in Printed circuit
solder.
Summary of Contents for LOGIQ V2
Page 8: ...i 6 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Page 92: ...Getting Started 1 80 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Page 242: ...After the Exam is Over 3 80 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Page 288: ...Safety 4 46 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Page 380: ...Index 4 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Page 381: ......