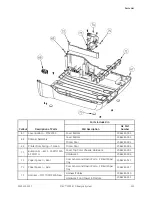
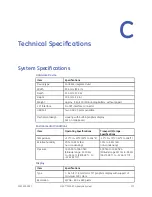
B
Electromagnetic Compatibility (EMC)
This section describes the EMC information relevant to your product.
Changes or modifications to this system not expressly approved by GE Healthcare
could cause EMC issues with this or other equipment. This system is designed and
tested to comply with applicable regulations regarding EMC, and must be installed
and put into service according to the EMC information stated in this section.
WARNING:
EQUIPMENT MALFUNCTION Use of portable phones or other radio frequency (RF)
emitting equipment near the system may cause unexpected or adverse operation.
Do not use portable phones or other electronic equipment that may emit radio
frequency (RF) near this system.
WARNING:
EQUIPMENT MALFUNCTION Do not use the equipment or system adjacent to, or
stacked with, other equipment.
If adjacent or stacked use is necessary, test the equipment or system to verify
normal operation in the configuration in which you are using it.
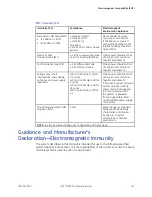
Guidance and Manufacturer’s
Declaration—Electromagnetic Emissions
The system described in this manual is intended for use in the following specified
electromagnetic environment. It is the responsibility of the customer or user to ensure
that this system is used in such an environment.
2053535-003C
MAC™ 2000 ECG Analysis System
165
Содержание MAC 2000
Страница 41: ...Product Overview 2053535 003C MAC 2000 ECG Analysis System 41 ...
Страница 134: ...Maintenance 134 MAC 2000 ECG Analysis System 2053535 003C ...
Страница 160: ...Parts List 160 MAC 2000 ECG Analysis System 2053535 003C ...
Страница 182: ...182 MAC 2000 ECG Analysis System 2053535 003C ...
Страница 183: ......