Page 12 P/N 03521015 Rev U1
Bio-Medical Devices Intl
17171 Daimler Street Irvine, CA 92614 800-443-3842 www.maxair-systems.com
Refer to the regulatory approval inserts shipped with your systems, and the following NIOSH website addresses for MAXAIR
CAPR regulatory approval status.
http://www2a.cdc.gov/drds/cel/cel_results.asp?startrecord=1&Search=cel_form&maxrecords=50&manufacturer=BMD&appdatefrom=&appdate
to=&powered=&scbatype=&scbause=&privatelabel=
http://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/PAPRtables.html
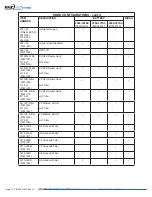
Table 2. summarizes current components that may be used to configure systems relative to the applicable Approval Body.
CUFF AND SHROUD CONFIGURATIONS
ITEM
NUMBER DESCRIPTION
CARTRIDGE FILTER
NIOSH
2164-10
HE
(01031327)
2163-10
XP
(01031279)
2167-10
XP
(01031569)
2166-10
XP
(01031593)
2081-03
(P/Ns
03531001,
03531021,
01031269,
2590-05)
Helmet with Cage
w/o Cage
w/o Cage
w/o Cage
w/o Cage
2083-03
(P/Ns
03531001,
03531148,
01031269,
2590-05)
Helmet with Cage and HH Liner
w/o Cage
w/o Cage
w/o Cage
2071-06
(03531021)
Helmet Liner (Standard)
2071-05
(03531148)
Helmet Liner (Hard Hat)
2061-08
(01031284)
Filter Cover Cap (FCC)
2061-04A
(01031528)
Hard Hat
2400-090L
(03521128)
Impact Lens
2365-02ML
(01031272)
DLC Lens-Cuff, Medium-Large
2365-02SM
(01031316)
DLC Lens-Cuff, Small
2366-02ML
(01031555)
DLC Lens-Cuff, Medium-Large, HH
2366-02SM
(01031556)
DLC Lens-Cuff, Small, HH
3.2 Approved System Configuration Components
Table 2. Approved System Components
BATTERY CHOICE(S)
PER
FILTER CHOICE
(01532104)
2500-
36TSC
36TSC
(01532161)
37TSC
37TSC
37TSC
(01532116)
30TSC
30TSC
30TSC
30TSC