Chapter 8 Technical Specifications
8.1
Specifications
81
8
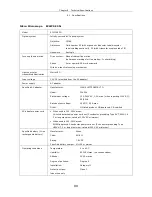
Transport/storage
conditions
Temperature: -20
to
60
°
C
Humidity:
90% RH max. (no condensation)
External dimensions and
weight (main unit)
External dimensions:
184 (W) x 358 (H) x 383 (D) mm (excluding projections)
Weight: Approx.
8.5
kg
Safety standards
•
UL-listed product (UL61010A-1)
•
Meets FCC Part 15B Class A requirements.
This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful
interference when the equipment is operated in a commercial environment.
This equipment generates, uses, and can radiate radio frequency energy and, if not
installed and used in accordance with the instruction manual, may cause harmful
interference to radio communications.
Operation of this equipment in a residential area is likely to cause harmful
interference in which case the user will be required to correct the interference at his
own expense.
•
This Class A digital apparatus complies with Canadian ICES-003.
Cet appareil numérique de la classe A est conforme à la norme NMB-003 du
Canada.
•
Complies with Australian EMI (AS/NZS2064 Group1 Class B).
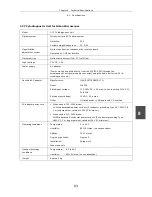
CE Marking
•
Meets EU IVDD (In vitro diagnostic medical device Directive) requirements.
(GM-approved: in vitro diagnostic medical device)
•
Meets EU Low Voltage Directive requirements.
•
Meets EU EMC Directive (EN61326) requirements.
•
UL-listed and GS-approved certification were obtained for the following
combination: 55i, cytodiagnostic unit, hand switch, specified adapter, and
specified battery.