PN 9000-280-VE, Rev B
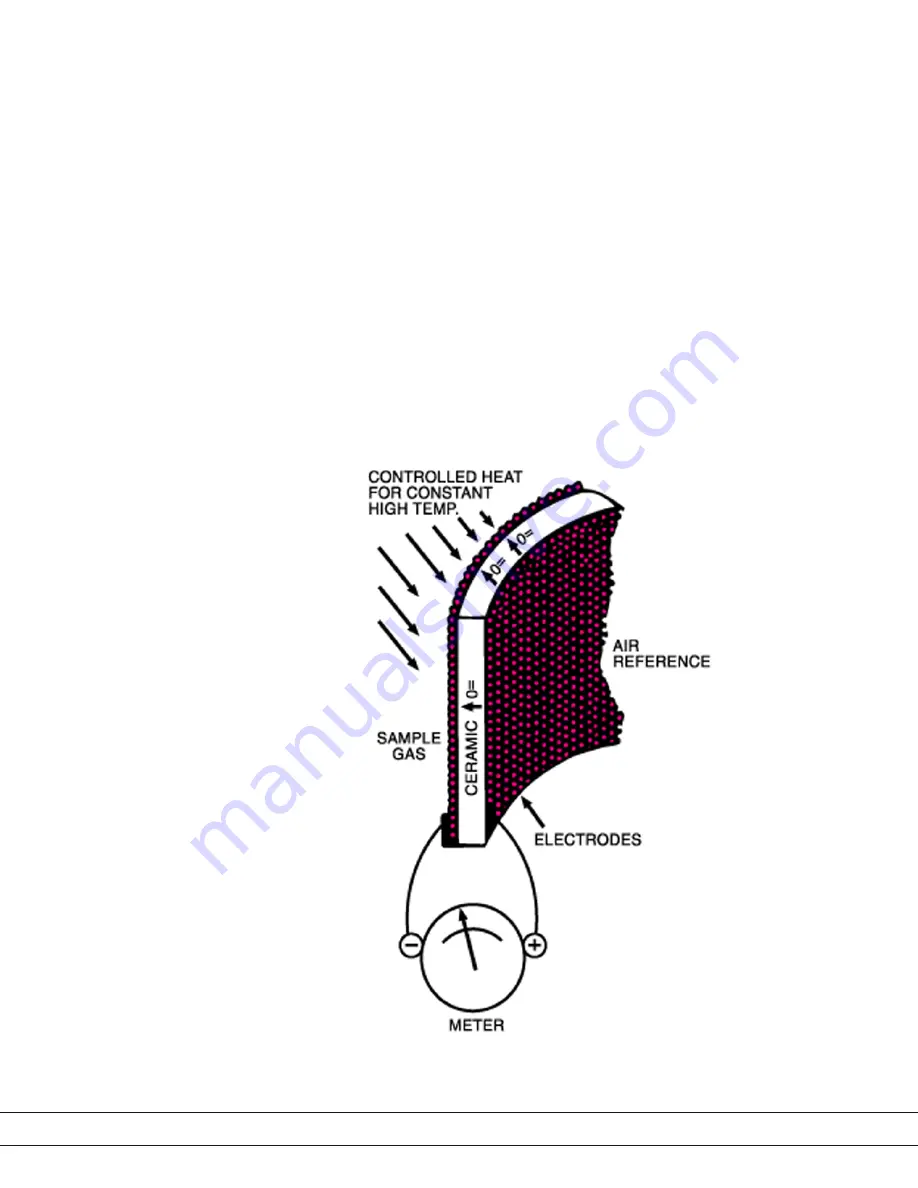
How the Oxygen Measuring Cell Works
The sensing element is a closed-end tube made from ceramic zirconium oxide
stabilized with an oxide of yttrium or calcium. Porous platinum coatings on the
inside and outside serve as both a catalyst and as electrodes. At high tempera-
tures (generally above 650 °C/1200 °F), oxygen molecules coming in contact
with the platinum electrodes near the sensor become ionic.
• As long as the oxygen partial pressures on either side of the Cell are equal,
the movement is random and no net flow of ions occurs.
• If, however, gases having different oxygen partial pressures are on either
side of the Cell, a potentiometric voltage is produced. The magnitude of
this voltage is a function of the ratio of the two oxygen partial pressures.
• If the oxygen partial pressure of one gas is known, the voltage produced
by the Cell indicates the oxygen content of the other gas. A reference gas,
usually air (20.9 % O
2
), is used for one of the gases.
Figure 1-1.
Zirconium oxide Cell principle
of operation.
1-6 | Thermox
®
WDG-V UOP ATEX-IECEx Analyzer
Содержание WDG-V UOP
Страница 1: ...WDG V UOP Combustion Analyzer ATEX IECEx USER MANUAL PN 9000 280 VE_Rev C...
Страница 40: ...PN 9000 280 VE Rev B Figure 2 7 Conduit Entries 2 18 Thermox WDG V UOP ATEX IECEx Analyzer...
Страница 96: ...PN 9000 280 VE Rev B WDG V UOP Series Analyzer Rating Labels 4 6 Thermox WDG V UOP ATEX IECEx Analyzer...
















































