CARDIO LINE 400/400 MED
Technical and optical modifications as well as misprints reserved -
© 2011
by ERGO-FIT GmbH & Co. KG
6 3
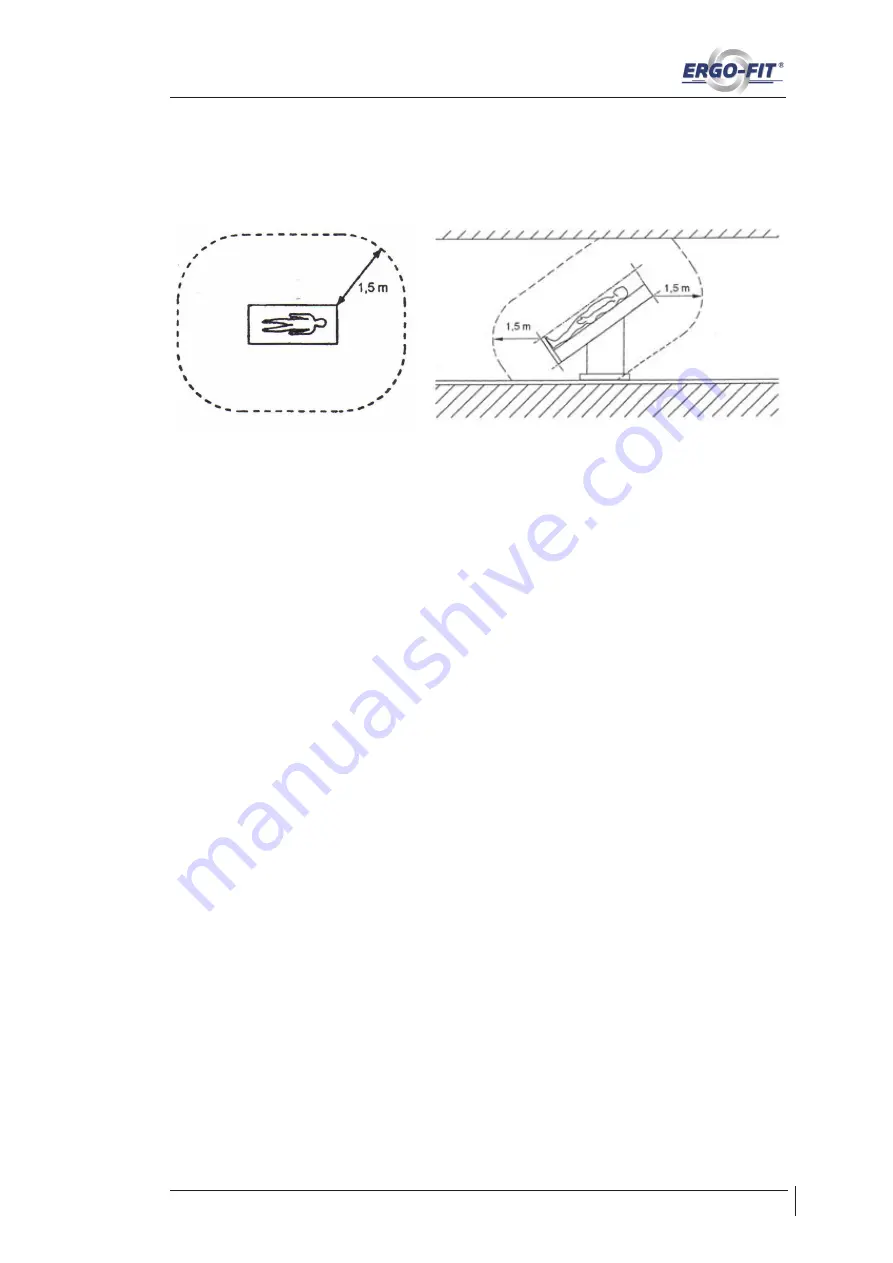
The distance to be maintained is 1.5 metres as a practical value. With this distance,
two training devices cannot be connected conductively by a person and it is unlikely
that users will receive an electric shock while training.
The instructions given in this chapter refer to the safety model as it is recognised in
Germany. These instructions may vary in other countries.
A.5.2
Mark of conformity
The CE mark attached to the cardio machine (CYCLE 407/457 MED only) refers to
the EMC conformity (electromagnetic conformity in compliance with EMC Directive
2004/108/EC, Medical Device Directive 93/42/EEC as well as Low Voltage Directive
2006/95/EC). The CE mark attached to the cardio machine (CYCLE 400/450 without
MED) refers to the EMC Directive 2004/108/EC and the Low Voltage Directive
2006/95/EC. The tests are based on interference emission and interference immunity
parameters.
The ERGO-FIT exercise machines of the CARDIO LINE 400 MED are manufactured
in accordance with the strictest safety and quality standards and are designed for
commercial use.
The CYCLE models of the CARDIO LINE 400 series are manufactured in compliance
with the guideline 2004/108/EEC (EMC), the requirements of the standards EN 957
and EN 60335-1 and are designed for home use.
The CYCLE models of the CARDIO LINE 400 MED series are manufactured in
compliance with the guidelines 93/42/EEC (MDD) and 2004/108/EC (EMV) as well as
the requirements of the standards EN 957, DIN VDE 750-238, EN 60601-1 and EN
60601-1-2.
Furthermore, all CYCLE models of the CARDIO LINE 400/400 MED are subjected to
the standard DIN 62304.