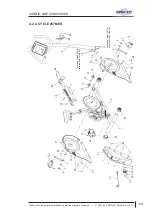
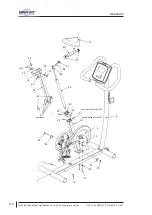
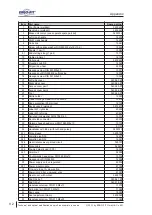
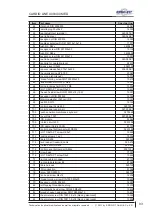
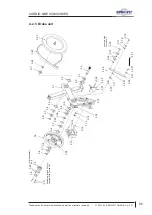
Appendix
Technical and optical modifications as well as misprints reserved -
© 2011
by ERGO-FIT GmbH & Co. KG
1 0 2
EG-Konformitätserklärung
EC Declaration of conformity:
CYCLE 407 MED
CYCLE 457 MED
fulfil the requirements laid down in the following standards and guidelines
• 2004/108/EC
EMC (electromagnetic compatibility) Directive
• MPG, MDD 93/42/EEC, class of models IIa
Medical Device Directive
• EN 957
Stationary training equipment
• DIN EN 60601-1, class of protection I, degree of protection type B
Medical electrical equipment – Part 1: General requirements for safety and essen-
tial performance
• DIN EN 60601-1-2
Medical electrical equipment – Part 1: General requirements for safety and essen-
tial performance – Collateral standard: Electromagnetic compatibility - Require-
ments and tests
• DIN EN 60601-1-6
Medical electrical equipment – Part 1: General requirements for safety and essen-
tial performance – Collateral standard: Usability
• DIN VDE 0750-238
Medical electrical equipment – Part 238 Particular requirements for the safety of
crank ergometers
• DIN EN 62304
Medical device software - Software life-cycle processes
Notified body:
DQS GmbH
August-Schanz-Straße 21
60433 Frankfurt am Main
Kennnummer 0297
This declaration applies to all machines supplied from 01.01.2011 to 31.12.2011
and has been issued with full responsibility to the producer
ERGO-FIT GmbH & Co. KG
Blocksbergstraße 165
66955 Pirmasens
by:
Michael Resch
(Managing Director)
Pirmasens, 08.02.2011