16.2.2 Salt bridge Ag/AgCl reference electrode
The Ag/AgCl reference electrode with salt bridge consists of a silver rod, coated with solid AgCl,
immersed in a solution of saturated KCl containing KCl crystals. Electrical contact with the other
electrodes in the flow cell is made through a salt bridge consisting of a wetted cotton wool frit,
which is electrically conducting and slows down leakage of KCl. This REF for the VT-03 flow cell
is filled with KCl at the factory. For certain applications, another chloride salt may be preferable.
With perchlorate-containing mobile phases, sodium chloride is mandatory, because potassium
perchlorate precipitates and will clog the cotton wool frit. At high modifier percentages, the REF
must be filled with lithium chloride for similar reasons.
16.2.3 HyREF reference electrode
The HyREF is a hydrogen reference electrode. Its potential depends on the pH of the mobile
phase. The HyREF is comparable to the standard Ag/AgCl REF in baseline stability and S/N
ratio. The HyREF is more user-friendly and, in principle, requires no maintenance. Trapping of air
bubbles, like in the salt bridge Ag/AgCl type, is impossible because of the absence of a salt
bridge. Consequently, refilling the REF with saturated KCl is not required. Due to the absence of
a salt bridge and its inertness, the HyREF is an excellent alternative to the Ag/AgCl REF,
especially in high modifier concentrations (as in analysis of fat-soluble vitamins) or high pH (as in
analysis of carbohydrates, PAD).
Depending on the pH of the mobile phase, the potential setting of the working electrode vs. the
HyREF may differ significantly compared with Ag/AgCl. I/E curves show a shift of more than 200
mV at pH 3.1 (for example, catecholamines), but no shift appears at pH 12 (for example, PAD of
carbohydrates). It is advisable to first construct a hydrodynamic (or scanning) voltammogram
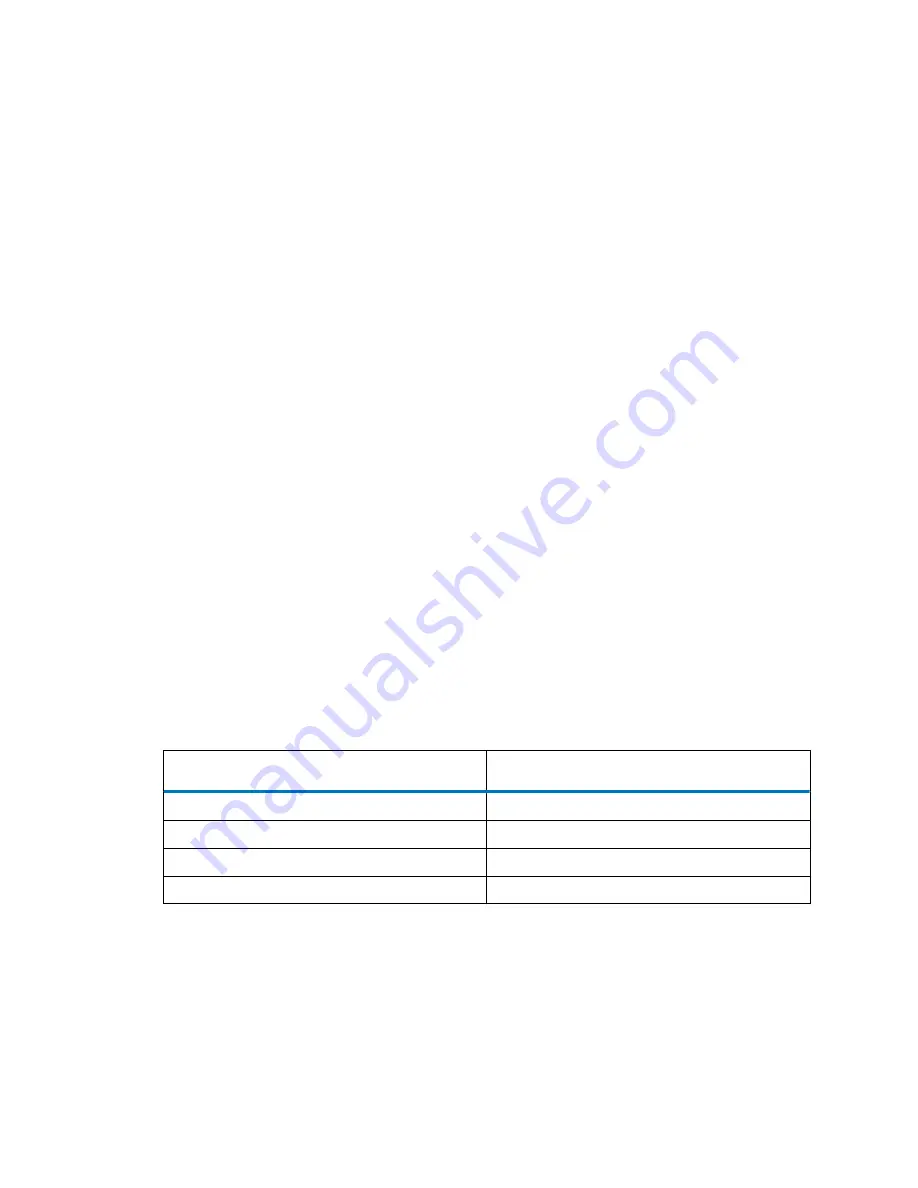
when using the HyREF. In the following "Measured cell potential (HyREF - Ag/AgCl) versus pH"
table, the potential of the HyREF is measured against the Ag/AgCl (in saturated KCl) electrode at
different pH values.
Table 16–6: Measured cell potential (HyREF - Ag/AgCl) versus pH
pH
E
HyREF-Ag/AgCl
(mV)
3.3
232
6.2
130
7.5
90
11.8
0
So, if an Ag/AgCl REF is replaced by a HyREF, the pH effect must be taken into account (refer to
the "Measured cell potential (HyREF - Ag/AgCl) versus pH" table). The pH vs. voltage relation is
described by: E
HyREF
= E
Ag/AgCl
- 328 + 29.9 pH
Example: A working potential of 800 mV (vs. Ag/AgCl with saturated KCl) at pH 3 must be
changed to E
HyREF
= 800 - 328 + 29.9*3 = 561.7 mV (vs. HyREF).
December 16, 2021, 715007395 Ver. 00
Page 163






























