11
SOLUTION
Cu°
Cu + 2e
++ -
HOCI + 2e
-
+ -
CI + OH
CATHODE
ANODE
1.7
Galvanic Cell Theory
Water, in pure form, is relatively nonconductive, but addition of an ionizing species (e.g. salt)
allows current to pass. The greater the concentration of ions in solution, the greater the
conductance of the solution.
If two electrodes are immersed in an ion containing solution, a chemical species capable of being
reduced (gaining electrons) can move toward the cathode where electrons are transferred from
the cathode to the reducible species, resulting in a cathodic current.
At the same time, an oxidation reaction (where an oxidizable species loses electrons) occurs at
the anode. Electrons are then transferred to the anode, producing an anodic current.
As the reaction occurs at the cathode, the
concentration of the reducible species at the
cathode drops. In response to the resultant concentration
gradient created, more of the reducible species moves
toward the cathode. This is referred to as diffusion. The
speed of movement of the reducible species toward the
cathode is called the rate of arrival.
The rate that the reducible species arrives at the cathode is
dependent on its concentration. As the concentration
increases the diffusion to the cathode increases, which
increases the current.
The current is also affected by temperature. With elevated
temperatures, diffusion increases. Most systems
compensate for temperature in some fashion.
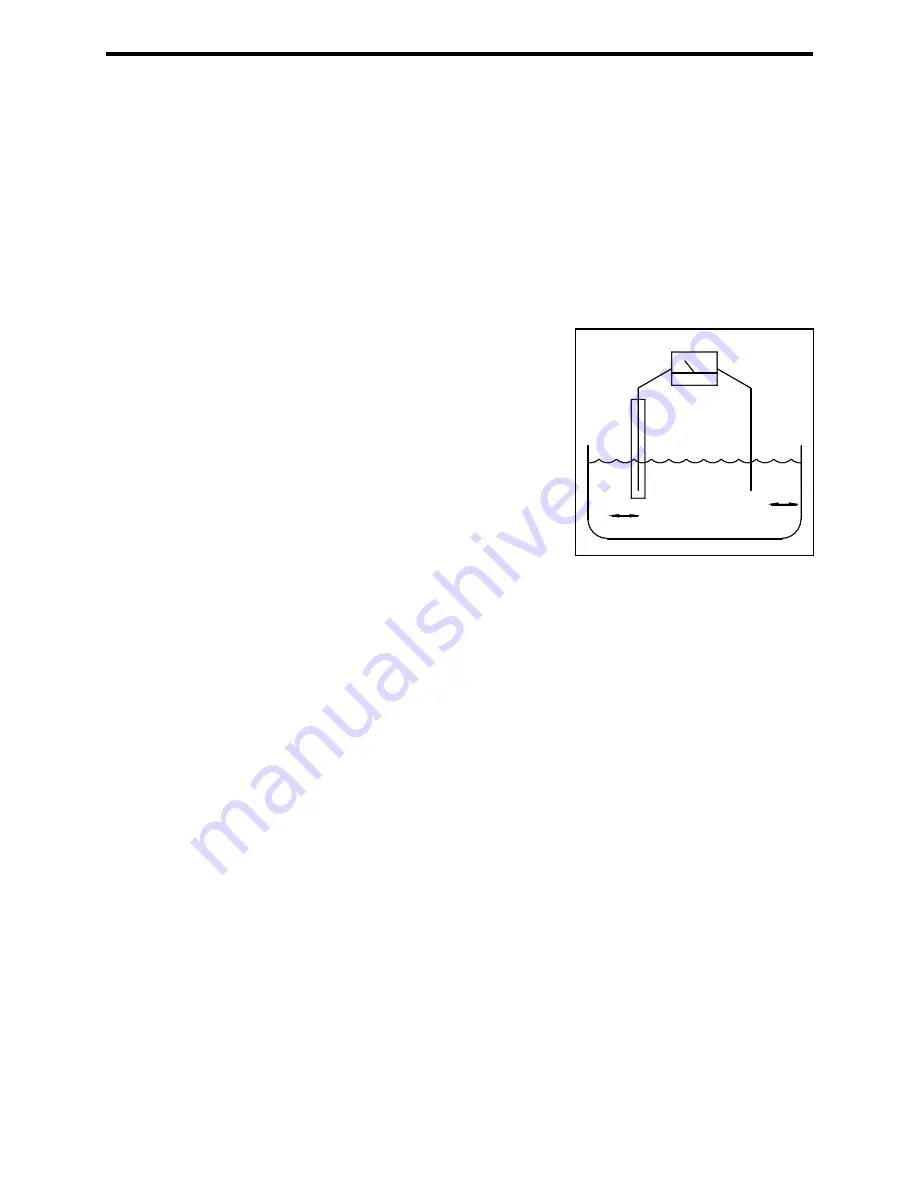
Figure 7 – Galvanic Cell
If the electrodes are made of two (selected) dissimilar metals and the proper conditions exist with
regard to solution composition, current can be produced by simply connecting (shorting) the
electrodes. This type of cell is called a galvanic cell.
In a galvanic cell, a change in concentration is detected by measuring the change in current
flowing through the cell (Reference Figure 7). The cell current responds proportionally to changes
in concentration.
The cathode in the galvanic cell used in the Cl1000B Chlorine Analyser is gold. When
hypochlorous acid (or hypochlorite ion) is present in solution, electrons are exchanged at the
cathode surface and chloride ions are produced.
HOCl + 2e
-
→
Cl
-
+ OH
-
The anode is copper. As electrons are exchanged, an oxide product remains on the anode.
Because of this, an abrasion mechanism (constant stirring of cleaning spheres) is incorporated to
strip the oxide product off the metal surface. Since copper is consumed in the process, the term
sacrificial anode is applied to the copper electrode.
In addition to the gold cathode and copper anode, the Cl1000B includes a third, reference
electrode. The reference electrode fixes the potential across the cathode to provide an accurate,
stable residual indication.
Current flow in the amperometric cell may be affected by large changes in pH.
Temperature compensation circuitry is employed to counter the effects of diffusion and other
factors that are affected by temperature.
Copper Gold












































