Device Labels
LOGIQ V2/LOGIQ V1
–
User Guide
4-37
Direction 5610736-100
Rev. 9
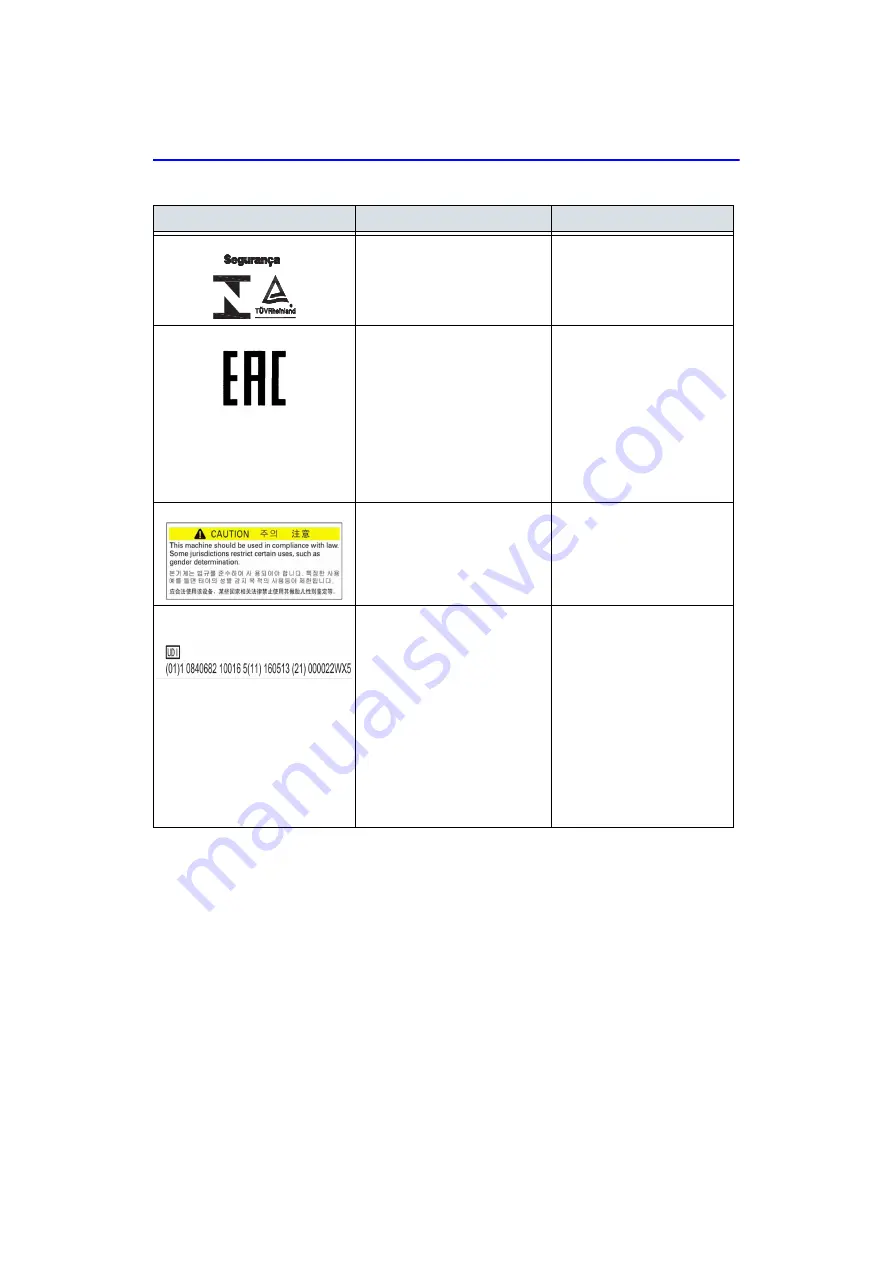
INMETRO Certification: TUV
Rheinland Brazil
Rating plate
Note: Only after Brazilian
regulatory registration is
complete, this label will be
located on the console.
“Eurasian Conformity” mark;
thesingle conformity mark
forcirculation of products on
themarkets of member-states
ofCustoms Union.
This product passed allconformity
assessment(approval)
procedures thatcorrespond to the
requirementsof applicable
technicalregulations of the
CustomsUnion.
Bottom.
This machine should be used in
compliance with law. Some
jurisdictions restrict certain uses,
such as gender determination.
Bottom of the system
Note: For China, Korea and
India only
Every system has a unique
marking for identification, the
Unique Device Identification
(UDI) Label. The UDI label
consists of a series of
alpha-numeric characters and
barcode which uniquely identify
the LOGIQ V2/LOGIQ V1 system
as a medical device
manufactured by General
Electric. Scan or enter the UDI
information into the patient health
record as required by
country-specific laws.
Rating plate
Table 4-6: Label Icons (Continued)
Label/Icon
Purpose/Meaning
Location
Содержание LOGIQ V2
Страница 4: ...i 2 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9 This page intentionally left blank...
Страница 8: ...i 6 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Страница 92: ...Getting Started 1 80 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Страница 170: ...After the Exam is Over 3 8 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9 Figure 3 10 Formats selection...
Страница 242: ...After the Exam is Over 3 80 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Страница 288: ...Safety 4 46 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Страница 380: ...Index 4 LOGIQ V2 LOGIQ V1 User Guide Direction 5610736 100 Rev 9...
Страница 381: ......