7
POTENTIAL ADVERSE EVENTS
Potential adverse events associated with ERCP include, but are not limited to:
pancreatitis, cholangitis, cholecystitis, cholestasis, aspiration, perforation, hemorrhage,
infection, liver abscess, sepsis, allergic reaction to contrast or medication, hypotension,
respiratory depression or arrest, cardiac arrhythmia or arrest.
Additional adverse events that can occur in conjunction with biliary stent placement
include, but are not limited to: trauma to the biliary tract or duodenum, perforation,
obstruction of the pancreatic duct, stent migration, stent occlusion, tumor ingrowth
or excessive hyperplastic tissue ingrowth, tumor overgrowth, stent misplacement,
pain, fever, nausea, vomiting, inflammation, recurrent obstructive jaundice, bile duct
ulceration, death (other than due to normal disease progression).
MRI SAFETY INFORMATION
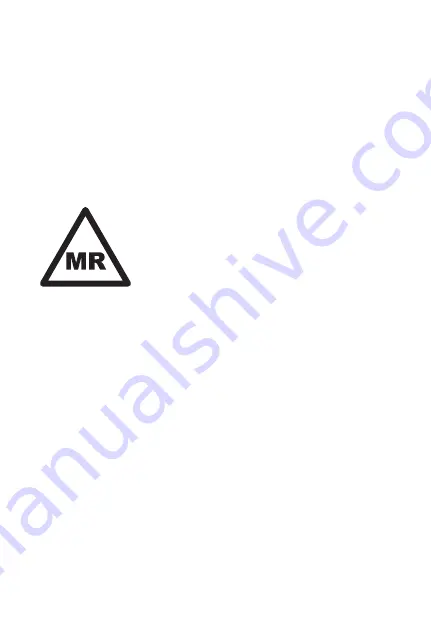
This symbol means the device is MR conditional
Nonclinical testing has demonstrated the Zilver 635® Biliary Stent is MR Conditional
(for either a single stent, a pair of overlapping stents or a pair of side-by-side stents). A
patient with this device can be safely scanned in an MR system meeting the following
conditions:
• Static magnetic field of 3 T or 1.5 T
• Maximum spatial field gradient of 1900 gauss/cm (19 T/m)
• Maximum MR system reported, whole body averaged specific absorption rate (SAR)
of 2 W/kg (Normal Operating Mode)
Under the scan conditions defined above, nonclinical testing results indicate the Zilver
635® Biliary Stent is expected to produce a maximum temperature rise of less than 5.0°C
after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately
14 mm from the Zilver 635® Biliary Stent when imaged with a spin echo pulse sequence
and a 3 T MR system.
For US Patients only
Cook recommends that the patient register the MR conditions disclosed in this IFU
with the MedicAlert Foundation. The MedicAlert Foundation can be contacted in the
following manners:








































