ColonyDoc-It Imaging Station
44
Supporting 21 CFR Part-11 Compliance
Purpose
US - Food and Drug Administration (US-FDA) created and released Part 11 of Title 21 of Code of Federal
Regulations (CFR) in August 1997.
The rules delineate the conditions under which the US-FDA considers electronic records and electronic signatures
equivalent to paper records and paper signatures. The instructions for compliance really span the entire
organization and its practices. LS software by UVP is one piece that rightly fits into the bigger picture and
supports
compliance.
Note:
While software from UVP, LLC is an essential tool for assisting an organization to maintain CFR
c
ompliance, UVP cannot claim that this is the only tool needed to achieve overall CFR compliance. The
organization must establish policies and procedures that work in conjunction with such efficient tools, to
ensure total compliance with 21 CFR Part 11 regulations.
Features
UVP provides software support for the following two sections of CFR regulations:
Section 11.10 (e) – For electronic records, this section requires the use of computer- generated, time-
stamped audit-trails to track changes.
The software keeps track of all changes that affect image-data. Any action in the software that modifies the
original data of an image open in the LS workspace, is logged. The log of such changes is individually
maintained for each image and is referred to as „History
‟
in the software.
Section 11.3 (b) (4) – This section mandates that the system be controlled by users responsible overall for
contents of electronic records required to track.
The software provides an elaborate system of maintaining secure user accounts. Assign unique usernames and
passwords to all the users who will be using the software. Each account can also be configured to provide read or
modify access to other users
‟
data. Events generated in the audit trail (above) are logged with the username.
Usage
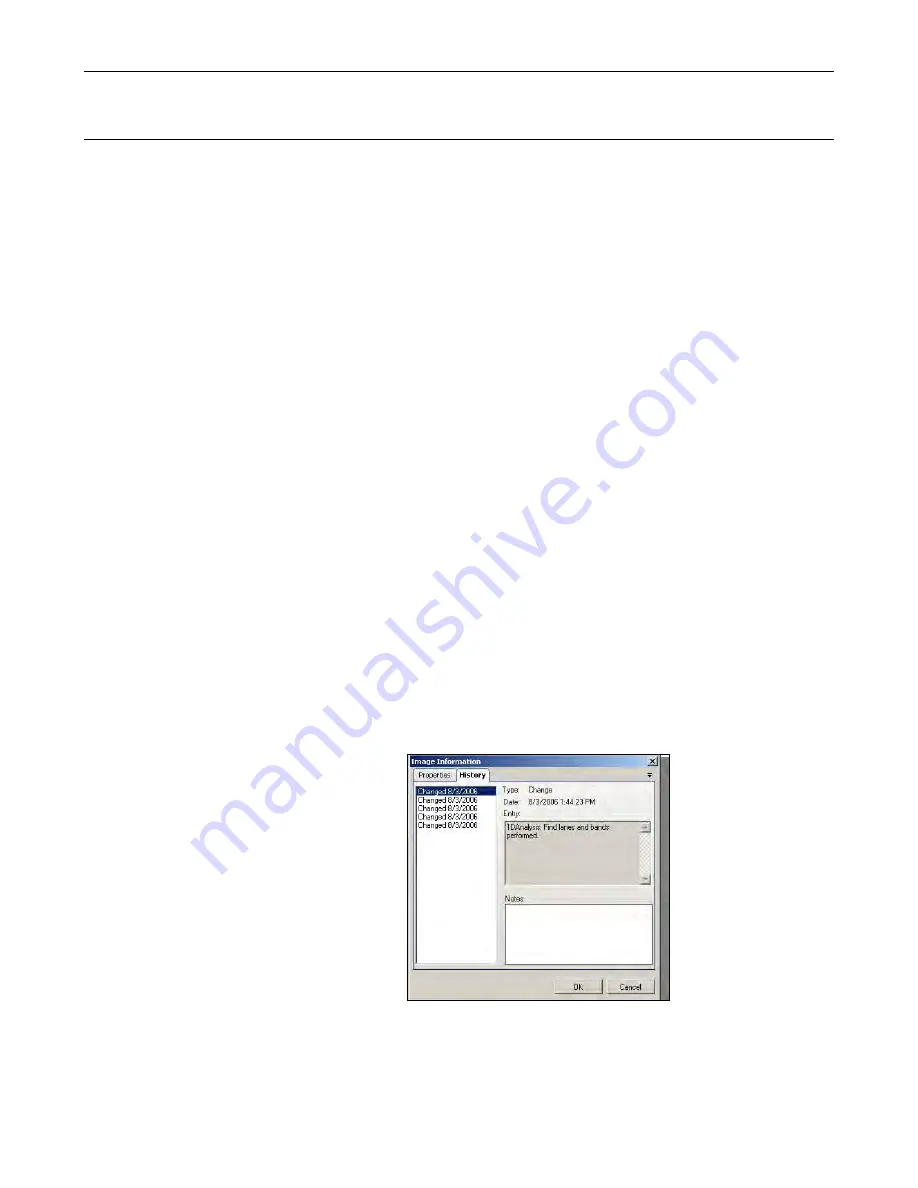
View an Audit Trail (History)
Open the image in question.
Right click on the image and select
Image Information. Open the
History
tab.
Events are listed in the left column.
Click on each event to view the entry
details on the right.
Add notes to each event if required.





































