Technical note
87
●
ORP measurement
ORP is an abbreviation for oxidation-reduction potential. ORP is the energy level
(potential) determined according to the state of equilibrium between the oxidants (M
z+
)
and reductants (M
(z-n)+
) that coexist within a solution.
For one type of equilibrium in a solution:
...............................................①
If only
①
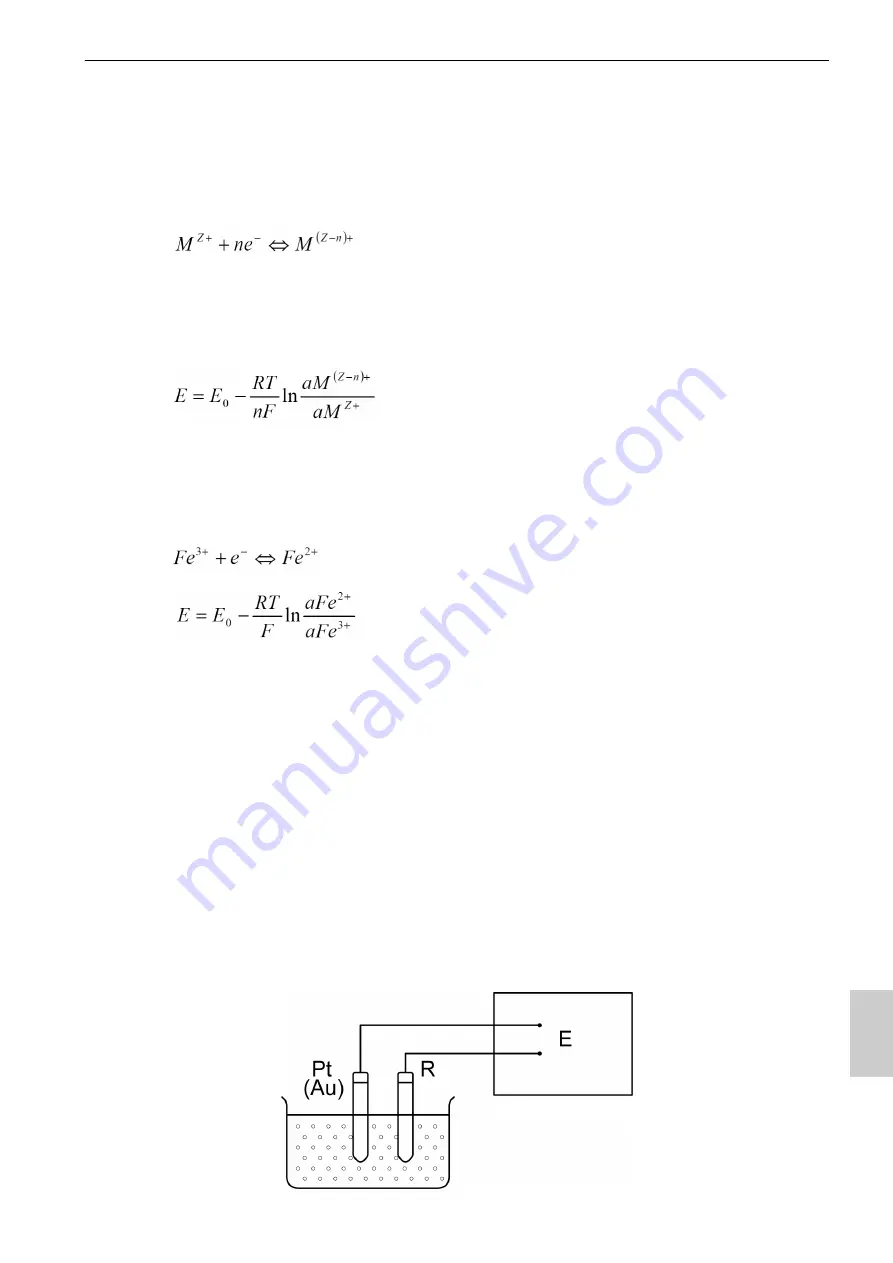
exists within a solution, a metal electrode (platinum, gold, etc.) and a reference
electrode are inserted into the solution, forming the ORP measuring system shown in
Fig. measuring the potential (ORP) that exists between the two electrodes enables the
potential to generally be expressed by the following equation.
.........................................②
E: Electric potential, E0: Constant, R: Gas constant, T: Absolute temperature
n: Electron count, F: Faraday constant, a: Activity
For example, for a solution in which trivalent iron ions coexist with bivalent iron ions,
equations
①
and
②
would be as follows.
.......................................................①
'
...........................................②
'
When only one type of equilibrium state
①
exists in the solution, the ORP of the solution
can only be determined by equation
②
. What is important here is that ORP is determined
by the ratio of activity between the oxidant (Fe
3+
) and the reductant (Fe
2+
) (using the
equation aFe
2+
/aFe
3+
). In actuality, however, many kinds of states of equilibrium exist
simultaneously between various kinds of ions, in most solutions. This means that under
actual conditions, ORP cannot be expressed using the simple equation shown above
and that the physical and chemical significance with respect to the solution is not very
clear. In this respect, the value of ORP must be understood to be only one indicator of
the property of a solution. The measurement of ORP is widely used, however, as an
important index in the analysis of solutions (potentiometric titration) and in the disposal
and treatment of solutions.
Recently, various claims have appeared regarding this matter. For example, that a high
degree of ORP is effective in sterilization, or that drinking water that has a low ORP
reduces the chance of illness by reacting with the activated oxygen in the cells of the
body. ORP is used as an index for alkaline drinking water.
Potentiometer
Содержание LAQUAact D-75G
Страница 2: ......
Страница 22: ...M E M O...
Страница 88: ...M E M O...
Страница 94: ...M E M O...
Страница 116: ......
Страница 117: ......
Страница 118: ......










































