Technical note
88
●
Dissolved oxygen measurement
Dissolved oxygen (DO) is the concentration of oxygen that is dissolved in water. DO is
essential in the self-cleaning mechanism of rivers and seas and for fish and other aquatic
animals. The measurement of DO is also essential for waste-water treatment and water-
quality management.
The principles of measurement using a DO tip are explained below.
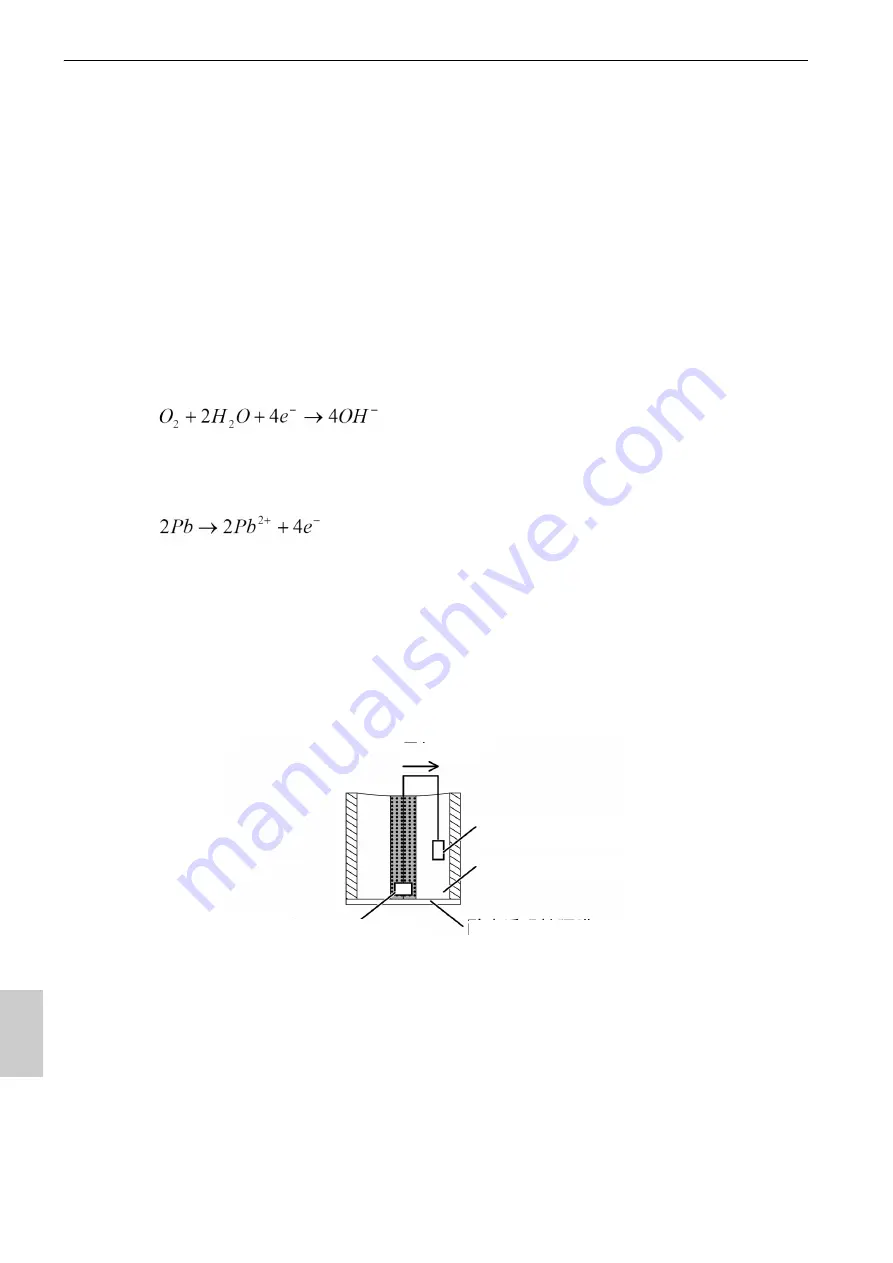
A precious metal (silver) is used as the cathode, which is tightly affixed to an oxygen-
permeable membrane, and a base metal (lead) is used as the anode. Both the cathode
and anode are immersed in an alkaline electrolytic solution. The external circuit between
the anode and cathode is closed. Oxygen that diffuses through the oxygen-permeable
membrane causes the following chain reaction to occur in the cathode and allows current
to flow in the external circuit.
Whereas, the following oxidation reaction occurs at the anode.
This current is proportional to the amount of oxygen that is diffused through the oxygen-
permeable membrane, so measuring the current of the sample enables the DO
contained within the sample to be determined. The DO measurement method that is
based on this principle is called the “Membrane electrode method.” This is a much
simpler and more convenient way of measuring DO than using chemical analysis, which
requires complex pretreatment in order to eliminate the effects of reductants and
oxidants in the sample.
Anode (lead)
Alkaline electrolyte
Gas-permeable membrane
Electrode
Cathode (silver)
Содержание LAQUAact D-75G
Страница 2: ......
Страница 22: ...M E M O...
Страница 88: ...M E M O...
Страница 94: ...M E M O...
Страница 116: ......
Страница 117: ......
Страница 118: ......









































