Page 15
/
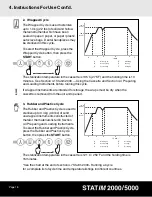
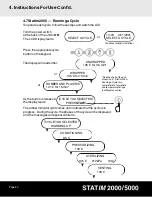
4. Instructions For Use Cont’d.
• All Instruments
The Statim is
NOT
intended for sterilizing textiles, liquids or biomedical waste.
Unwrapped instruments, once exposed to ambient or external
conditions, cannot be maintained in a sterile state. If sterile storage is desired, wrap
the instruments to be sterilized in autoclave bags, according to the instrument
manufacturer’s instructions. Then allow the wrapped cycle to run until the air-dry
phase is complete.
Best Practice
: Allow instruments (wrapped or unwrapped) to dry completely prior to
handling. In order to promote drying and enable effective sterilization, wrapped or
pouched instruments must not touch each other.
• Note for Ophthalmology Use
In the field of ophthalmology, proper wrapping or pouching of surgical instruments will
reduce the exposure of instruments to any process residues during the sterilization
cycle. Due to the highly sensitive nature of some types of surgery (particularly in
ophthalmology), SciCan recommends that all instruments be routinely packaged or
wrapped and processed through the wrapped cycle of the sterilizer. This practice is the
suggested approach for the majority of sterile surgical procedures and is referenced in
most leading infection control publications and guidelines.
• Routine Monitoring
Chemical process indicators suitable for steam sterilizers should be included in or on
each package or load being sterilized. In addition, the weekly use of biological
indicators, which allow you to ascertain whether the instruments have been exposed to
sterilization conditions, is recommended. For Statim 5000 units in the United States,
SciCan recommends using the 3M Attest™ biological monitoring system for routine
monitoring. This system consists of self contained biological indicators and incubators.
It is important to select the correct biological indicator for the cycle being tested.