RespiraSense
™
Instructions For Use
RespiraSense
™
PDS-801-007 Revision 2
Page
67
of
77
S
ECTION
10
–
A
SSOCIATED
IT
E
QUIPMENT
The following section provides information about the associated IT equipment of the RR Monitor.
Note
The mobile medical software application is installed onto the hand-held device by
the manufacturer.
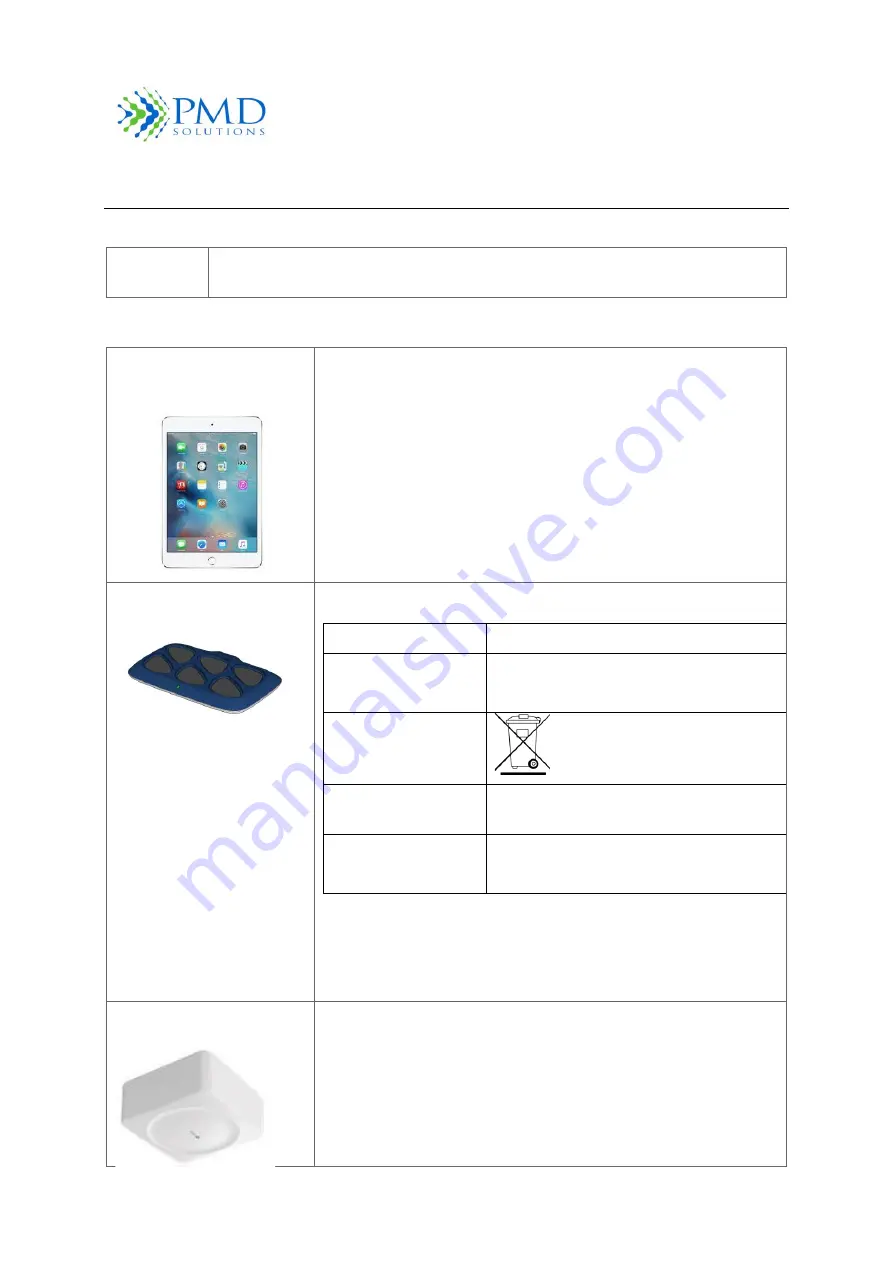
Table 18 List of Associated IT Equipment
iPad Mini 4 or 7
th
Gen or
above
PDS-701-000
Approved Mobile device for RS Device Mobile Medical Applications
Multi Charging Dock
PDS-102-000
Charging Dock for RS Devices
Power Supply
25 W 5 VDC / 5 A
Output
0.5A / 5V x 6 ports
4A / 5V x 1 port (USB)
WEEE
CE
Power Supply and charging station are CE
marked
Power source
Mains-Powered
90-264VAC~50/60Hz 1A MAX
●
The charger is to be used outside of the patient
environment.
●
The charger is to be commissioned by IT professionals.
●
The charger should be operated by trained personnel.
Air Repeater Module
PDS-901-000
WiFi to Bluetooth repeater for enabling RS Device Air Dashboard











































