25
Chemistry
Method:
1. Place the vessel into the freezer. (Make sure that the cable cannot get ‘trapped’ in the door)
2. Start logging.
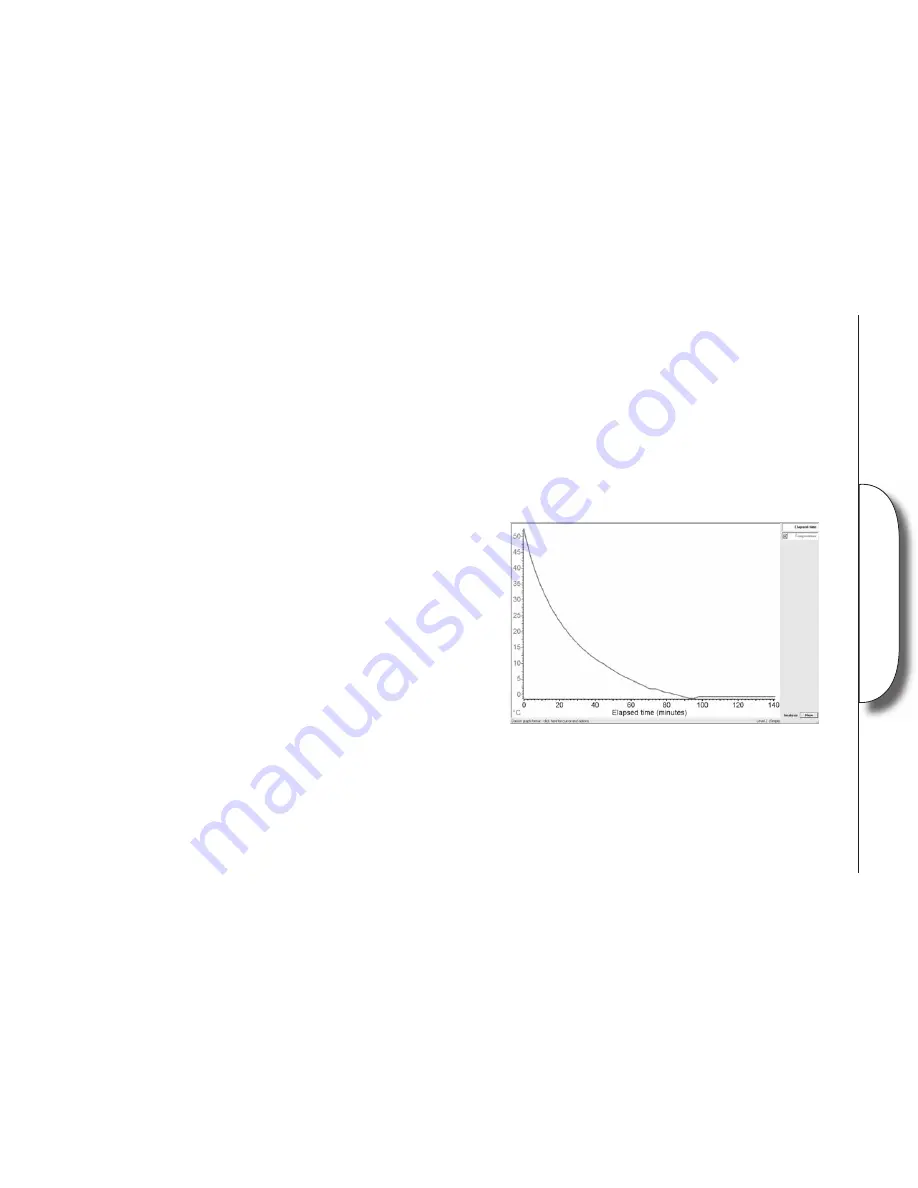
3. Allow the water to freeze. (the time needed depends on the amount of water used)
4. Stop logging.
5. Repeat the experiment using distilled water or salty water.
6. When fi nished the results can be saved and printed.
Results:
Did the cooling curve give the expected result?
What factors might have affected the fi nal curve?
What is signifi cant about the shape of the cooling curve?
How do the distilled, salty and tap waters compare? Why is this so?
Why might the tap water not freeze at 0
0
C?
Going further:
Try different starting temperatures of water.
Try using more than one temperature sensor and have different
concentrations of salt.
Calculate the rate of temperature change for the cooling curves.
Try other liquids such as pure orange juice or squash.
Why might this experiment be useful to freezer manufacturers or food
companies?
















































