19
Method:
1. Carefully pour hot water into the inner small container.
2. Carefully pour cold water into the larger outer container to the same level as the inner container.
3. Place the temperature sensors into the two containers making sure they do not touch the sides.
4. Start the datalogging software.
5. While logging the experiment can be discussed with students asked to predict what will happen. Students could sketch a graph.
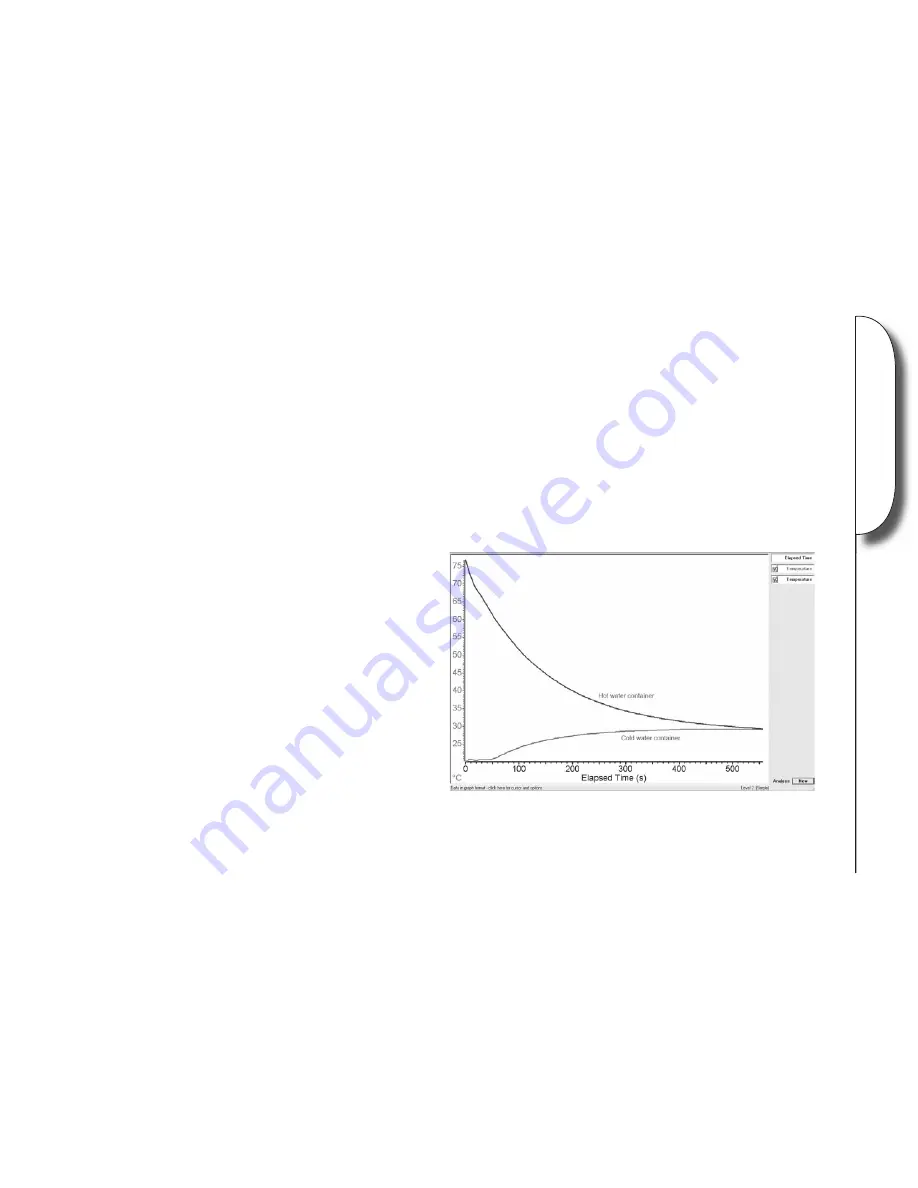
6. Once the temperatures no longer rise or fall, stop the datalogging software and save or print the results.
Results:
Looking at the two traces, where did the heat energy appear to go?
What would happen to the two temperatures if the experiment was left for a few hours?
When did the cold water gain heat energy and then lose it?
What happens to the heat energy lost by the cold water in the outer container?
Why is it important not to let the temperature sensors touch the sides?
Going further:
Leave the cooling process over a longer period of time.
Put lids on the containers. What effect might this have?
What about the shape and volume of the containers?
Try insulating the outer container. What effect might this have?
Physics






























