6000-300-000 4–1
4 – Operation
WARNINGS
BLOOD AND BODY FLUIDS MAY CONTAIN THE HEPATITIS B VIRUS (HBV),
HEPATITIS C VIRUS (HCV), HUMAN IMMUNODEFICIENCY VIRUS (HIV), OR OTHER
DISEASE-CAUSING AGENTS. HANDLE ALL PATIENT SPECIMENS AS POTENTIAL
BIOHAZARDS CAPABLE OF TRANSMITTING INFECTION. WEAR APPROPRIATE
PERSONAL PROTECTIVE EQUIPMENT, INCLUDING LABORATORY GLOVES,
WHEN COLLECTING, HANDLING, AND PROCESSING BLOOD AND BODY FLUIDS.
IN ADDITION TO WEARING GLOVES, THE USE OF DISPOSABLE LAB COATS OR
GOWNS AND PROTECTIVE GLASSES OR GOGGLES IS RECOMMENDED WHEN
WORKING AROUND THE INSTRUMENT.
ACRIDINE ORANGE REAGENT MAY BE TOXIC; DO NOT INGEST. AVOID CONTACT
WITH SKIN, EYES, AND CLOTHING.
IF A TUBE BREAKS IN THE UNIT, CAREFULLY REMOVE THE TUBE WITH A HE-
MOSTAT OR OTHER DEVICE, USING PUNCTURE RESISTANT UTILITY GLOVES.
CONTACT TECHNICAL SUPPORT FOR ADDITIONAL INFORMATION.
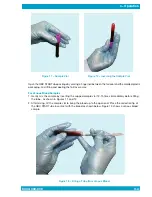
Summary of Operation Steps
Routine operation of the QBC STAR Hematology System consists of the following steps:
• Turn the instrument on
• Print out / save electronic controls
• Fill tube with blood
• Mix the tube of blood
• Place the cap onto the tube
• Place the tube into the instrument
• Close door and ensure it is latched
• Push the “STAR” button to start the test cycle
• Obtain the results from the printer
• Dispose of the QBC STAR tube in a biohazard sharps container
Each of these steps is described below.
6000-300-000 4-1
Summary of Contents for QBC STAR
Page 14: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 28: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 40: ...QBC STAR System Operator s Manual 6000 300 000 4 12 THIS PAGE INTENTIONALLY LEFT BLANK...
Page 50: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 72: ...6000 300 000 A 1 8 Appendices 6000 300 000 8 1...
Page 74: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 76: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 78: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Page 80: ...THIS PAGE INTENTIONALLY LEFT BLANK...