7
pH
Electrode
Reference
Element
Filling
Solution
Filling
Solution
Aperture
Membrane
Washer
Body
Body
Seal
Washer
Membrane
End Cap
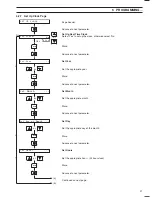
Sample
Inlet
Three Channel
Peristaltic Pump
To Electronics Section
Drain via
Constant
Head Unit
Heat Exchanger
Block
Sample
Heater Coil
Ammonia
Probe
Sample / Calibrate
Solenoid Valves
EDTA
Reagent
Solution
Orange
Channel
Standard
Solution 1
(Low)
Reaction
Coils
Constant Head Unit
Orange
Channel
Heater
Mat
Standard
Solution 2
(High)
NaOH
Reagent
Solution
Red
Channel
Red
Channel
SV1
SV2
Overflow
Drain
Contaminated
Drain
Out of Sample
Float Switch
From
Flowcell
Temperature
Sensor
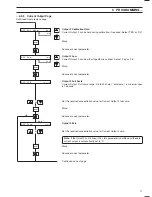
Fig. 4.2 Flow Schematic
Note.
In applications in the power
industry where hardness is very low, the
first reagent (EDTA) is not required.
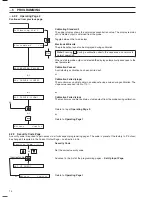
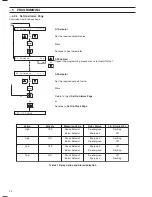
Fig. 4.1 Ammonia Probe
4
LIQUID HANDLING SECTION
4.1
Principle of Operation – Fig. 4.1
The monitor uses an ABB Ammonia Probe. This contains a
glass pH electrode, whose pH-sensitive glass membrane forms
a slightly convex tip, and a robust, long-life reference electrode.
The two electrodes are combined into a single assembly, and
are connected as a pH measuring pair through an internal
reservoir of filling solution containing ammonium ions.
The filling solution is 0.1 M ammonium chloride saturated with
silver chloride and is separated from the sample by a gas-
permeable hydrophobic membrane fitted in the tip of the
probe. Sample is caused to flow past the probe membrane,
whereupon the partial pressures of ammonia gas in the two
solutions on either side of the membrane equilibrate,
transferring gas across the membrane. At equilibrium, the
concentration of ammonia in the thin film of filling solution
between the probe membrane and the glass electrode
membrane equals that in the sample. The resultant change in
pH value of the thin film is measured by the pH electrode pair
which thus develops an output potential related to the
ammonia concentration in the sample. Like most ion-selective
electrodes, the Ammonia Probe produces an output which is
logarithmic with respect to concentration.
Range of measurement can be set to any two consecutive
decades of concentration between 0.05 to 2000 mg l
–1
as N,
NH
3
, or NH
4
+
.
Under typical circumstances, with appropriate standard
solutions and calibration frequencies, accuracies better than
±
5% of reading or 0.1 mg l
–1
whichever is the greater, can be
achieved.