Why do some gases absorb
infrared energy?
16
From a mechanical point of view, molecules in a gas could be compared to weights (the
balls in the figures below), connected together via springs. Depending on the number of
atoms, their respective size and mass, the elastic constant of the springs, molecules
may move in given directions, vibrate along an axis, rotate, twist, stretch, rock, wag, etc.
The simplest gas molecules are single atoms, like helium, neon or krypton. They have no
way to vibrate or rotate, so they can only move by translation in one direction at a time.
Figure 16.1
Single atom
The next most complex category of molecules is homonuclear, made of two atoms such
as hydrogen (H
2
), nitrogen (N
2)
and oxygen (O
2
). They have the ability to tumble around
their axes in addition to translational motion.
Figure 16.2
Two atoms
Then there are complex diatomic molecules, such as carbon dioxide (CO
2
), methane
(CH
4
), sulfur hexafluoride (SF
6
), and styrene (C
6
H
5
CH=CH
2
) (these are just a few
examples).
Figure 16.3
Carbon dioxide (CO
2
), 3 atoms per molecule
This assumption is valid for multi-atomic molecules.
Figure 16.4
Methane (CH
4
), 5 atoms per molecule
Figure 16.5
Sulfur hexafluoride (SF
6
), 7 atoms per molecule
Figure 16.6
Styrene (C
6
H
5
CH=CH
2
), 16 atoms per molecule
Their increased degrees of mechanical freedom allow multiple rotational and vibrational
transitions. Because they are built from multiple atoms, they can absorb and emit heat
#T559900; r. AB/35735/35735; en-US
43
Summary of Contents for G300 pt Series
Page 1: ...User s manual FLIR G300 pt ...
Page 2: ......
Page 3: ...User s manual FLIR G300 pt T559900 r AB 35735 35735 en US iii ...
Page 4: ......
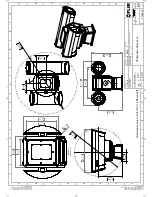
Page 42: ...Mechanical drawings 13 T559900 r AB 35735 35735 en US 36 ...
Page 44: ...CE Declaration of conformity 14 T559900 r AB 35735 35735 en US 38 ...
Page 45: ......
Page 79: ......