DIAMAG
Operating Manual
Page 21
Guidance and manufacturer
’s declaration –
electromagnetic emissions and immunity
Medical electrical equipment needs special precautions regarding
EMC and needs to be installed and put into service according to EMC in-
formation provided in this document.
Attention!
The use of mobile radio frequency media may affect
MEDICAL ELECTRICAL EQUIPMENT.
• The device must be used in the electromagnetic environment spec-
ified in Tables 1-4.
• Make sure that the equipment operates correctly if the conditions
are different from those shown in the tables below.
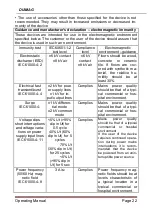
Guidance and manufacturer
’s declaration –
electromagnetic emissions
These devices are intended for use in the electromagnetic environment
specified below. The customer or the user of the device should assure that
the device is used in such an environment.
Emissions test
Compliance
Electromagnetic environment
— guid-
ance
RF emissions
CISPR 11
Group 1
The device uses RF energy only for
its internal function. Therefore, its RF
emissions are very low and are not
likely to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11
Class B
The device is suitable for use in all es-
tablishments, including domestic es-
tablishments and those directly con-
nected to the public low-voltage net-
work that supplies buildings used for
domestic purposes.
Harmonic Emis-
sions
IEC 61000-3-2
Class A
Voltage Fluctua-
tions/Flicker
Emissions
IEC 61000-3-3
Complies
WARNING
• The device should not be used adjacent to or stacked with other equip-
ment. If adjacent or stacked use is necessary, the device should be ob-
served to verify normal operation in the configuration in which it will be
used.