22
The combination of steps 1 to 3 are required to assure that the storage agents have been effectively
and sufficiently removed.
Step 1 WFI Flush, quickly removes the bulk of the storage agent, so only small quantities of water are
required in this step.
Step 2 is designed to reduce or eliminate bioburden from the cassette and to extract most of the
remaining storage agents.
Step 3 flushes out the sanitizing agent and reduces extractables to acceptable levels. Flushing volumes
must be determined by the user for the specific application.
The combination of steps 4 and 5 establish the fitness of the cassette for use or reuse.
Step 4 measurement of Normalized Water Permeability (NWP) is required to establish a pre-use baseline
value against which the cassette can be measured following its use in a process and
subsequent cleaning. Comparing the initial NWP to the NWP value measured after cleaning is a
way to establish the effectiveness of the cleaning process.
Step 5 establishes the integrity of the cassettes and system against leaks and possible product loss.
Step 6 buffer conditioning, prepares the wetted surfaces of the system as well as the membrane in the
cassette before the addition of process fluid by removing trapped air from the system and
equilibrating the system in the process buffer to reduce the risk of product precipitation or
denaturation. It also equilibrates the system temperature to the product temperature.
Details of each step are presented in the following sections.
Warning: Exceeding the maximum operating pressure for the cassette can permanently damage the
cassette.
4.1
Using a Cassette for the First Time
First time use of new Omega membrane cassettes requires the removal of the storage agents
(such as sodium hydroxide) from the cassettes. A material safety data sheet with important
information about the preservative agents is included with each cassette.
The volume of flushing agent and flushing time to achieve the required minimum level of
extractables may be greater for first time use of cassettes compared to subsequent use and
will depend on the porosity of the membrane and the temperature of the flushing solution.
The procedures outlined in Sections 4.2, 4.3 and 4.4 are designed to efficiently and effectively
remove these agents but may need to be modified to meet your specific requirements for trace
level contaminants.
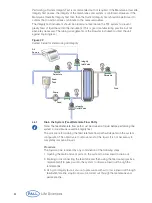
4.2
Initial Flushing of the Cassette and Assembly (WFI Flush)
Put the cassette in holder. Use 2 X 1/16 silicone gaskets cleaned with DI water. Flush the
cassette and hardware assembly with pharmaceutical-grade water. Use of a lower quality water
to flush the system may introduce inorganic impurities that could affect membrane performance
and product recoveries.
Water Quality for Flushing:
WFI (water for injection) at 25 °C or
0.2 µm filtered DI water at 25 °C
Volume Required:
10 L/m
2
(1 L/ft
2
)
4.2.1
Flush the Feed/Retentate and Permeate Line to Waste
Direct the retentate and permeate line to waste.