39
Results of CLIA Waiver Study
A total of nine untrained non-laboratory users from three clinical sites in the US participated in the
study by testing a total of 560 urine samples using the
AimStrip
®
Urine Analyzer and the
CTMI
CT-120 Analyzer over 2 month period. The
AimStrip
®
Urine Analyzer is intended for use in
conjunction with the
AimStrip
®
and
Fisherbrand
®
Urinalysis Reagent Strips for detection of the
following analytes in urine: Glucose, Blood, Protein, Leukocyte, Nitrite, Bilirubin, Ketone, pH,
Specific Gravity, and Urobilinogen. At each study site, approximately 120 clinical urine specimens
were tested by three untrained, non-laboratory participants: phlebotomist, medical assistants, and
ultrasound technician. The results (WM) were compared with the same specimens tested by
professionally trained operators using Bayer’s Clinitek 500 Urine Chemistry Analyzer (CM).
The CLIA waiver study demonstrated that the following performance data of the
AimStrip
®
Urine
Analyzer in the hands of non-technical users when using only the Quick Reference Guide.
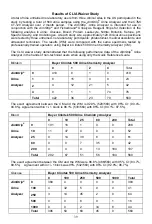
Bilirubin
Bayer Clinitek 500 Urine Chemistry Analyzer
0 1 2 4
Total
0
410
8 1 0 419
1
8 15
0 0 23
2
4 12 21 5 42
AimStrip
®
Urine
Analyzer
4
0 1 1 74
76
Total
422
36
23 79
560
The exact agreement between the CM and the WM is 92.9% (520/560) with 95% CI: (90.4%;
94.8%). Agreement /- 1 block is 96.1% (538/560) with 95% CI: (94.1%; 97.5%).
Blood
Bayer Clinitek 500 Urine Chemistry Analyzer
0
10
25
80
200
Total
0
191 16 1 0 0
208
10
11 37 4 0 0 52
25
0 14
39
8 0 61
80
0 0 7 35
0 42
AimStrip
®
Urine
Analyzer
200
0 0 0 13
184
197
Total
202
67
51
56
184
560
The exact agreement between the CM and the WM were 86.8% (486/560) with 95% CI: (83.7%;
89.5%). Agreement /- 1 block was 95% (532/560) with 95% CI: (92.9%; 96.7%).
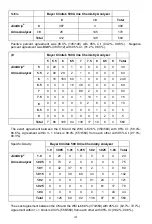
Glucose
Bayer Clinitek 500 Urine Chemistry Analyzer
0
100
250
500
1000
Total
0
389
4 0 0 0 393
100
4 32
5 0 0 41
250
3 14 45 2 0 64
500
0 0 8 9 1 18
AimStrip
®
Urine
Analyzer
1000
0
0
2
34
8
44
Total
396
50
60
45
9
560