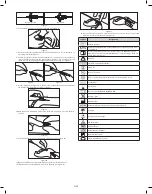
radiopaque
catheter
vent plug
FloSwitch
push tab
securement platform
notch needle
DESCRIPTION
The Merit RadialFlo™ arterial catheter is designed to access the vasculature to sample
blood and monitor blood pressure. The device is equipped with a FloSwitch to limit blood
spill or control flow as needed. The needle includes a unique notch to provide instant
blood return upon vessel entry. The radiopaque catheter provides visibility under X-ray
and fluoroscopy.
INTENDED USE / INDICATION FOR USE
The Merit RadialFlo™ arterial catheter is a device that is inserted into the patient’s vascular
system for short term use (less than 30 days) to sample blood and monitor blood pressure.
The Merit RadialFlo™ arterial catheter may be used for any patient population with
consideration given to adequacy of vascular anatomy and appropriateness of procedure.
REUSE PRECAUTION
For single patient use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or
resterilization may compromise the structural integrity of the device and/or lead to device
failure which, in turn, may result in patient injury, illness or death. Reuse, reprocessing or
resterilization may also create a risk of contamination of the device and/or cause patient
infection or cross-infection, including, but not limited to, the transmission of infectious
disease(s) from one patient to another. Contamination of the device may lead to injury,
illness or death of the patient.
CONTRAINDICATION
RadialFlo™ is for arterial blood pressure monitoring and arterial blood sampling only. Do
not use for arterial administration of intravenous infusions.
CLINICAL BENEFITS
• Allow blood pressure monitoring
• Allow blood sampling
WARNINGS
• Non-pyrogenic. Sterile, do not use if unit package is opened or damaged. Check
integrity of the individual package before use.
• For single use only. Dispose of product after use. Do not resterilize. Reuse may lead
to infection or other illness/ injury.
• Do not bend the needle when using the product.
• Do not use scissors / or sharp tools at or near the insertion site to avoid accidental
catheter shear.
• Do not attempt to re-insert a partially or completely withdrawn needle into the
catheter. If arterial puncture is unsuccessful discard the entire device.
• Exposure to blood, either through percutaneous puncture with a contaminated
needle or via mucous membranes, may lead to serious illness such as hepatitis, HIV,
AIDS, or other infectious disease.
• Physicians must be familiar with arterial access using over the needle technique.
• Physicians must be familiar with the complications associated with arterial
catheterisation, i.e. arterial ischema, air embolism, catheter fragmentation which
required additional procedure.
Federal Law (USA) restricts this device to sale by or on the order of a physician.
PRECAUTIONS
• RadialFlo™ inserted in emergency situations where sterile technique could be
compromised should be replaced within 48 hours. Follow hospital/institutional
protocols or procedures on recommended indwelling time for RadialFlo™.
• Device lifetime specified as 96 hours based on CDC & Joint Commission Intl (JCI)
recommendation for replacement frequency
• Ensure the placement location of RadialFlo™ does not prevent collateral flow to the
extremity.
• Immediately dispose of the needle post insertion in appropriate sharps container.
• Report needlestick injuries immediately following established facility protocol.
MRI SAFETY INFORMATION
Non-clinical testing demonstrated that the RadialFlo™ is MR Conditional. A patient with
this device can be scanned safely in an MR system under the following conditions:
I N S T R U C T I O N S F O R U S E
RadialFlo
™
Arterial Catheter
• Static magnetic field of 1.5-Tesla and 3-Tesla, only
• Maximum spatial gradient magnetic field of 2,000-Gauss/cm (20-T/m)
• Maximum MR system reported, whole body averaged specific absorption rate
(SAR) of 2-W/kg for 15 minutes of scanning (i.e., per pulse sequence) in the Normal
Operating Mode
STORAGE CONDITIONS
Store in cool dry place away from direct sunlight.
GENERAL GUIDELINES
• The RadialFlo™ Arterial Catheter is available in one size as below specification:
• G
au
ge Size: 20G
• Standard outer diameter: 1.10mm
• Standard length: 45mm
• Flowrate: 49ml/min
• For proper use, clinicians must be familiar with and trained in the use of the
RadialFlo™.
• Observe infection control precautions on ALL patients.
• Aseptic technique, proper skin preparation, and continued protection of the
insertion site are essential.
• Examine the catheter insertion site frequently.
• If sutures are used to secure RadialFlo™, suture through the fenestrations in the
securement platform.
• This product does not contain DEHP, DIBP, DBP & BBP.
• After use, dispose the device in a manner consistent with standard protocols for
waste disposal.
• In the EU, any serious incident that has occurred in relation to the device should
be reported to the manufacturer and the competent authority of the applicable
Member State.
INSTRUCTIONS FOR USE
1. Prepare the patient aseptically according to hospital policy and protocol.
2. Inspect the package to ensure there is no damage and sterility is maintained. Refer
to the arrows on the device label to open the package (see Figure 1).
Figure 1
3. Remove the device from the package (see Figure 2).
Figure 2
4. Remove the protective needle cover in a straight outward motion (see Figure 3).
Figure 3
5. Ensure the FloSwitch is in the appropriate open position prior to needle insertion
(see Figure 4).
English
2/52