Innova 2000 Cardiovascular Imaging System
GE Medical Systems
Pre–Installation Manual
REV 5
pim 2337741–100
34
1-3
IEC601–1–2 Electromagnetic Standard Compliance & Documentation
1-3-1
GENERAL SCOPE
This equipment complies with IEC60601–1–2 Edition 2 EMC standard for medical devices.
The Innova system is suitable to be used in the electromagnetic environment, as per the limits &
recommendations described in the tables here after:
Emission Compliance level & limits (Table 16).
Immunity Compliance level & recommendations to maintain equipment clinical utility (see
Table 17, Table 18 and Table 19).
Note:
This system complies with above–mentioned EMC standard when used with supplied
cables up to maximum lengths referenced in the MIS MAPS or system cables interconnect
diagrams.
1-3-2
ELECTROMAGNETIC EMISSION
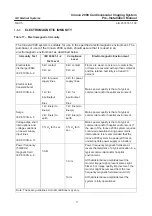
Table 16 – Electromagnetic Emission
The Innova 2000 system is suitable for use in the specified electromagnetic environment. The
purchaser or user of the Innova 2000 system should assure that it is used in an electromagnetic
environment as described below:
Emissions Test
Compliance
Electromagnetic Environment
Radio–Frequency
Emissions
CISPR11
Group1 Class A
limits
The Innova 2000 system is suitable for use in all
establishments other than domestic and those directly
connected to the low voltage power supply network that
supplies buildings used for domestic purposes.
Group1 Class A
limits
The Innova 2000 system
uses RF energy only for its internal
function. Therefore, the RF emission is very low and not likely
to cause any interference in nearby electronic equipment.
Harmonic emissions
IEC 61000–3–2
Not applicable
The Innova 2000 system is suitable for use only in
establishments not directly connected to a public low voltage
power supply network.
Voltage fluctuations/
flicker emissions
IEC 61000–3–3
Not applicable
The Innova 2000 system
is suitable for use only in
establishments not directly connected to a public low voltage
power supply network.