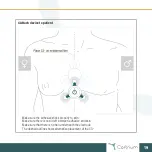
26
6.2 Regulatory information
The C3
+
is a class IIa medical device according to 93/42/EEC. The C3
+
complies with the following
product standards:
DS/EN 60601-1-1
Safety Requirements for Medical Electrical Systems
DS/EN 60601-1-2
Electromagnetic Disturbances
DS/EN 60601-1-6
Medical Electrical Usability
DS/EN 60601-1-11
Home Healthcare Environment
DS/EN 60601-2-47
Particular requirements for the basic safety and essential performan-
ce of ambulatory electrocardiographic systems
DS/EN 62366-1
Application of usability engineering to medical devices
DS/EN ISO 10993-1
Biological evaluation of medical devices
DS/EN ISO 15223-1
Symbols to be used with medical devices labels, labeling and
information supplied
DS/EN 1041
Information supplied by the manufacturer of medical devices