25
Biological
Medicinal Substances
N/A
Tissue
N/A
Body fluids contacted by device
N/A
Type of contact to intact skin
Non-invasive
Duration of skin contact
Up to 7 days continued contact
Mucosal membrane contact
N/A
Sterile or non-sterile
Non-sterile
Biological compatibility
ISO 10993-5
ISO 10993-10
Clinical
Medical purpose
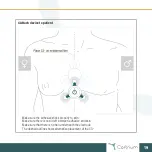
Ambulatory ECG
Single use / reusable
Reusable/Rechargeable Monitor
Recording standard
Holter
Recording format
Continuous
Intended placement
Midsternal line
Recording period
Up to 7 days on a single charge