5324310
6
02.24.18 PU 1295_34872049456.indd (SGS#4599854), update GB and prep for translations
kmh
02.28.18 Alts per marked up pdf
kmh
03.07.18 Collect InDesign files to be sent for translations
kmh
05.03.18 Update with new supplied translations.
TN
05.14.18 Alt per language
kmh
05.17.18 Final release.
TN
3
Monitoring Frequency
Follow facility Policies and Procedures which should
specify a biological indicator monitoring frequency
compliant with professional association recommended
practices and/or national guidelines and standards.
As a best practice and to provide optimal patient
safety, 3M recommends that every sterilization load be
monitored with a biological indicator.
Directions for Use
1. Remove 1295 BI from sealed foil pouch, then reseal
foil pouch if other 1295 BIs remain in foil pouch. Do
not place any labels or indicator tape on the vial or
on the cap.
2. Place the 1295 BI in a sterilization pouch indicated
for use in vaporized hydrogen peroxide sterilization
processes. Seal the sterilization pouch.
3. Place the pouched BI in the sterilizer chamber, with
the white side of the pouch facing up and the clear
plastic side facing down. When there is adequate
space in the loaded sterilizer chamber, place the
pouched BI directly on the sterilizer chamber rack
or shelf. The sterilizer manufacturer should be
consulted to identify the area of the chamber to
place the BI.
4. Process the load according to
recommended practices.
5. After completion of the cycle, don safety glasses
and gloves and remove the pouched BI from the
sterilizer. Inspect the 1295 BI to verify the media
ampoule is intact. If a chemical indicator was
included in the pouch with 1295 BI, inspect the
CI to assure the ink of the CI is not smeared or
runny. If the BI media ampoule is intact and the
ink of the CI (if included) appears typical remove
the BI from the sterilization pouch and proceed to
Step 6. If the media ampoule is broken or if the ink
of the CI appears smeared or runny, leave them
in the sterilization pouch and follow the disposal
instructions. Retest the sterilizer using a new 1295 BI
and CI (if included).
6. Check the process indicator on the top of the cap
of the 1295 BI. A color change of the stripes from
blue towards pink confirms that the 1295 BI has
been exposed to the vaporized hydrogen peroxide
sterilization process. This color change does not
indicate that the process was sufficient to achieve
sterility. If the process indicator is unchanged, check
the sterilizer physical monitors.
7. Identify the 1295 BI by writing the load number,
sterilizer, and processing date on the indicator label.
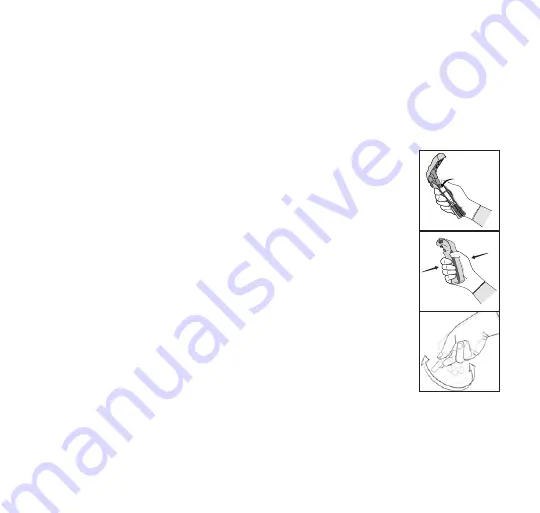
8. Activate the 1295 BI.
While wearing safety glasses
and gloves, place the 1295 BI
in an Attest™ Biological
Indicator Activator. Close
and squeeze the activator
to close the 1295 BI cap and
crush the media ampoule (see
pictures at right). Immediately
remove the BI and flick it
(see picture at right). Visually
verify that media has flowed
into the growth chamber at
the bottom of the vial. If the
media hasn’t filled the growth
chamber, hold the BI by the
cap and flick it until media fills
the growth chamber. Place
the activated 1295 BI in a
490H Auto‑reader incubation
well which is color‑coded
pink or 490 Auto‑reader
having software version
4.0.0 or greater incubation
well which is color‑coded
black and wait for the result.
See the Auto‑reader Operator’s Manual for further
information related to its use.
NOTE:
Activate and incubate the 1295 BI within
1 hour of the completion of the sterilization cycle.




















