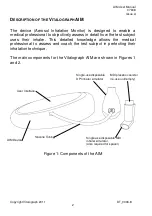
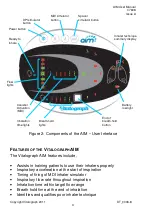
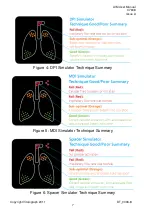
AIM User Manual
07608
Issue A
Copyright Vitalograph 2011 DT_0006-8
9
F
AULT
F
INDING
G
UIDE
Problem Fault
Symptoms:
•
Test begins automatically
•
Inhalation time accumulates automatically
without the subject inhaling.
Possible Causes:
(In probable
order)
•
Flowhead and/or tubing not stationary at the
start of test. Hold them steady until the ‘Blow
Icon’ appears.
•
Press the DPI, MDI or Spacer simulator button.
Problem Fault
Symptoms:
•
Rocking device
Possible Causes:
(In probable
order)
•
Check for damaged or missing rubber feet.
•
If any of the rubber feet are damaged or
missing replace all rubber feet.
Problem Fault
Symptoms:
•
No flow measurements.
Possible Causes:
(In probable
order)
•
Ensure that the silicone tubing is not pinched
or trapped.
•
Ensure that the silicone tubing is fitted to the
AIM device and the inhaler simulator.
Problem Fault
Symptoms:
•
Cannot read user interface.
•
Lights not coming on
Possible Causes:
(In probable
order)
•
The battery may be low. Replace the batteries.
•
Main PCB failure – contact support.
Problem Fault
Symptoms:
•
MDI simulator activation light not coming on.
Possible Causes:
(In probable
order)
•
Placebo canister is not fitted
•
Placebo canister is empty
Problem Fault
Symptoms:
•
Flow measurement appears low for the MDI