Cellartis iPSC rCas9 Electroporation and Single-Cell Cloning System User Manual
(030619)
takarabio.com
Takara Bio USA, Inc.
Page 4 of 24
I.
Introduction
The Cellartis iPSC rCas9 Electroporation and Single-Cell Cloning System allows efficient, footprint-free gene
editing of human induced pluripotent stem cells (hiPSCs) using the CRISPR/Cas9 system, followed by successful
clonal expansion of single, edited human iPSCs. Importantly, this system maintains karyotype and pluripotency
during the whole editing and culturing process.
The CRISPR/Cas9 system has emerged as a powerful tool for gene editing because of its high targeting
specificity, editing efficiency, and ease of use. The power of this technology derives from its simplicity, since all
it requires is a Cas9 nuclease enzyme combined with a single guide RNA (sgRNA) that determines its target
specificity (Jinek et al. 2012). This RNA-programmable method exploits the error-prone nature of the non-
homologous end joining DNA repair pathway (NHEJ) to generate gene knockouts (via insertion/deletion). The
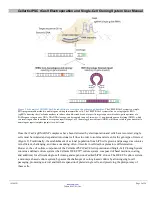
method can also be used to generate knockins via the homology-directed repair (HDR) pathway (Figure 1).
CRISPR/Cas9 system components have been delivered successfully into target cells through a variety of
approaches, including vector-based expression systems, transfection of RNA, and introduction of Cas9-sgRNA
ribonucleoprotein (RNP) complexes (Sander and Joung 2014). Delivery of Cas9-sgRNA RNPs via
electroporation, the method described in this manual, provides a fast turnaround for gene-editing experiments
while minimizing the likelihood of off-target effects compared to vector-based approaches (Kim et al. 2014).
The editing component of the Cellartis iPSC rCas9 Electroporation and Single-Cell Cloning System contains:
•
Recombinant wild-type Cas9 nuclease [Guide-it™ Recombinant Cas9 (Electroporation-Ready)]
o
This recombinant wild-type
Streptococcus pyogenes
Cas9 nuclease was expressed with a C-
terminal nuclear-localization signal (NLS) and purified from
E. coli
for use in CRISPR/Cas9-
mediated gene editing experiments.
o
The rCas9 protein solution has been verified to be sterile and well-tolerated by mammalian cells
when electroporated as an RNP with a sgRNA for knockout experiments, or as an RNP with a
donor repair template for knockin experiments.
•
The Guide-it sgRNA In Vitro Transcription Components v2 is used to produce high yields of sgRNAs
from
in vitro
transcription (IVT) reactions followed by the Guide-it IVT RNA Clean-Up Kit, used to
quickly purify sgRNA in a phenol-free manner.