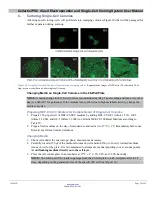
Cellartis iPSC rCas9 Electroporation and Single-Cell Cloning System User Manual
(030619)
takarabio.com
Takara Bio USA, Inc.
Page 24 of 24
4.
Resuspend the cells in 500 µl per well of pre-warmed DEF-CS SCC medium. Transfer all of the cell
suspension to a newly coated well in a 48-well plate.
NOTE:
To prevent cell loss,
counting the cells at this stage is not recommended.
5.
Tilt the dish backwards and forwards gently to ensure that the cell suspension is dispersed evenly
over the surface, then place it in an incubator at 37°C ± 1°C, 5% CO
2
, and >90% humidity.
X.
Scaling up from the 48-Well Plate
Once the cells have been passaged into a 48-well plate,
DEF-CS GF-3 is no longer needed in the growth
medium
used when changing the media. Prepare “DEF-CS medium for maintenance” by adding DEF-CS GF-1
(dilute 1:333) and GF-2 (dilute 1:1,000) to Cellartis DEF-CS 500 Basal Medium. When the cells are ready to be
scaled up to a 24-well plate, they can be cultured with the Cellartis DEF-CS 500 Culture System (Cat. No.
Y30010).
XI. References
Jinek, M.
et al.
A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity.
Science.
337,
(2012).
Kim, S., Kim, D., Cho, S. W., Kim, J. & Kim, J.-S. Highly efficient RNA-guided genome editing in human cells
via delivery of purified Cas9 ribonucleoproteins.
Genome Res.
24,
1012–9 (2014).
Sander, J.-D. & Joung, J.-K. CRISPR-Cas9 systems for genomic editing, regulation and targeting.
Nat.
Biotechnol.
32,
347–55 (2014).
For information on using the 4D Nucleofector System to electroporate cells, please visit:
http://www.lonza.com/products-services/bio-research/transfection/genome-editing.aspx
Contact Us
Customer Service/Ordering
Technical Support
tel: 800.662.2566 (toll-free)
tel: 800.662.2566 (toll-free)
fax: 800.424.1350 (toll-free)
fax: 800.424.1350 (toll-free)
web:
takarabio.com
web:
takarabio.com
e-mail:
e-mail:
Notice to Purchaser
Our products are to be used for research purposes only. They may not be used for any other purpose, including, but not limited to, use in drugs,
in vitro
diagnostic
purposes, therapeutics, or in humans. Our products may not be transferred to third parties, resold, modified for resale, or used to manufacture commercial products or to
provide a service to third parties without prior written approval of Takara Bio USA, Inc.
Your use of this product is also subject to compliance with any applicable licensing requirements described on the product’s web page at
takarabio.com
. It is your
responsibility to review, understand and adhere to any restrictions imposed by such statements.
© 2017 Takara Bio Inc. All Rights Reserved.
All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be
registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at
takarabio.com
.
This document has been reviewed and approved by the Quality Department.