SEN 257
Issue 2.1
06 October 2020
Page
4
of
29
8. Summary of Warnings and cautions
In common with all medical devices of this nature there are inherent risks and side effects.
Whilst every effort has been made to eliminate these risks, care should be taken when using
the device. It is important that the user familiarises himself/herself with all the warnings and
cautions contained within this document.
WARNINGS
The Philips monitor shows inoperable (INOP) messages to indicate that the TetraGraph device might be inoperable.
The user operates TetraGraph Monitor with TetraGraph User Interface at all times. Consult the IFU of TetraGraph for how to use
and handle the TetraGraph Monitor.
CAUTIONS
Please ensure that the TetraGraph Philips Interface is used with devices which it is intended to be used.
Please use the CAT5e cable that is supplied together with the TetraGraph Philips Interface. Any other cable may be incompatible
with the TetraGraph Philips Interface product.
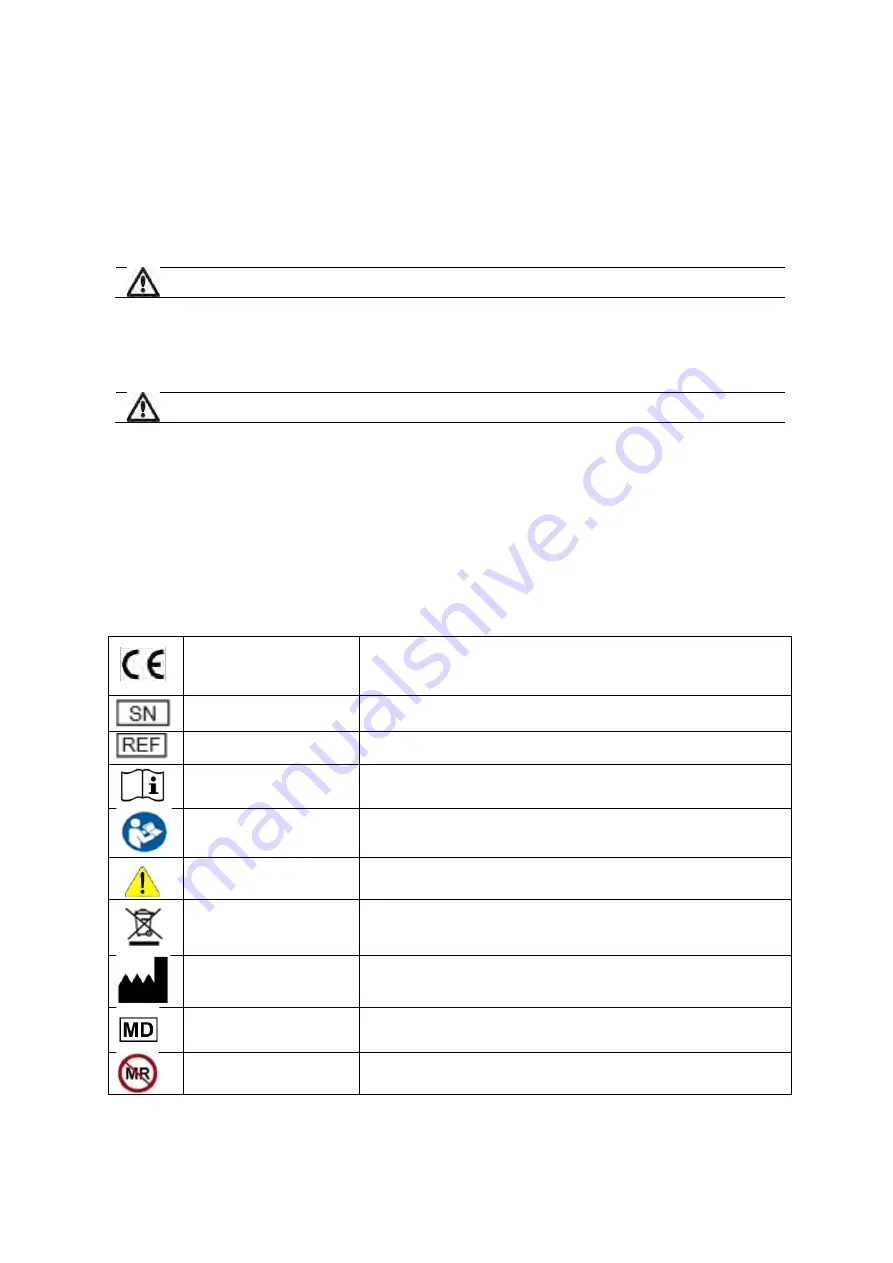
9. Symbols and Icons
The following symbols are used on the TetraGraph Philips Interface.
CE mark
Indicates compliance with the European Medical Device Directive 93/42 and
amendments thereto.
Symbol is associated with a number indicating the Notified Body.
Serial number
The unique serial number allocated to the device.
Reference number
The catalogue or model number of the device.
Operating instructions
The device has instructions for use.
Consult the instructions for use.
Refer to instruction manual
You must read the instructions for use.
General warning sign
Shows important information.
WEEE
Do not dispose of in domestic waste.
Manufacturer
Name and address of the manufacturer.
Medical Device
Medical Device
MR unsafe
The TetraGraph Philips Interface device is not MRI safe.