50
51
Experiment 9.9
The preparation of ammonia solution
• ammonium chloride
• calcium hydroxide
• 2 test tubes
• glass & rubber tubing
• cork for test tube with
hole
• beaker
• funnel
• test tube holder or
wooden clothes peg
• plastic test tube cap
• label
Note: This is the most difficult experiment in this booklet. Do not
try to do this experiment on your own, it is much easier if two
people do it together.
Ammonia gas is formed when ammonium chloride reacts with
calcium hydroxide.
The word equation for the reaction is:
ammonium ch calcium hydroxide a
calcium ch water.
Ammonia is VERY SOLUBLE in water. Because of this special
precautions must be taken when making it to ensure that no water
gets onto the hot solids. The funnel is used so that if any water is
sucked back it cannot get into the tube connecting the funnel to the
test tube containing the hot solids.
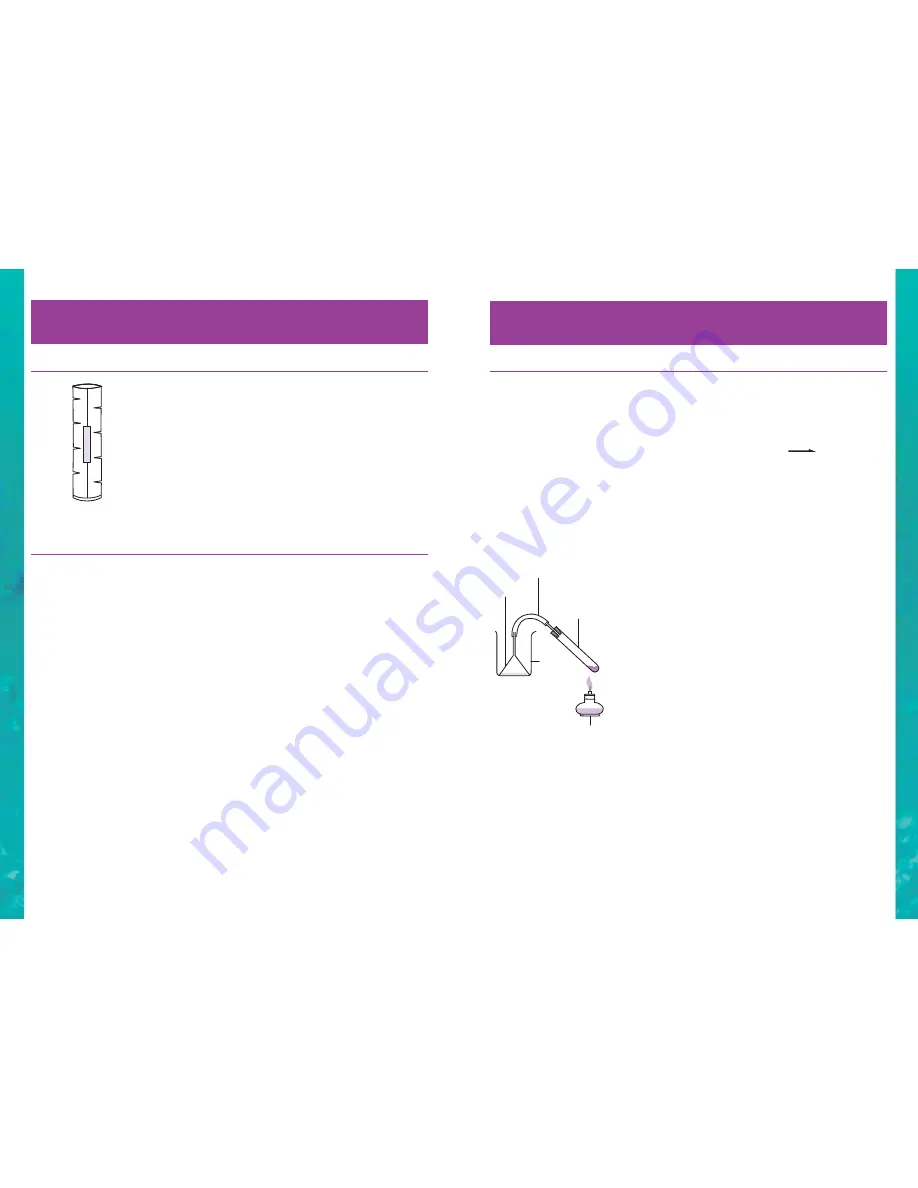
Assemble the apparatus as shown in the diagram. You need to cut
the “tag” off of the top of the funnel so that it will fit in the beaker.
Put ½ a test tube-full of water into the beaker.
Mix 3 measures of ammonium chloride with 2 measures of calcium
hydroxide on a piece of paper and carefully pour the mixture into a
clean dry test tube. Connect the test tube to the funnel as shown in
the diagram and arrange the apparatus so the solid reaction mixture
can be heated in the burner flame, the funnel is in the water in the
beaker, and the rubber tubing has no kinks in it. One person holding
the test tube with the test tube holder or a wooden clothes peg and
a second person gently holding the funnel in the beaker is best.
Heat the solid mixture in the flame. Fumes of ammonium chloride,
some of which will condense on the cold parts of the test tube, and
ammonia gas will be formed. The ammonia gas will dissolve in the
water under the funnel.
Continue heating for about 10 minutes, when there should be no
further change in the test tube. Remove the test tube from the flame
(put the HOT test tube on the tin tray) and the funnel from the water.
Pour the ammonia solution from the beaker into a test tube and put
a plastic cap on it. Label the tube and store it safely.
Experiment 9.7
The reaction of carbonates with acid
(continued...)
Carefully fill the packet with sodium hydrogen carbonate (sodium
bicarbonate) and when full, very carefully cut 4 “nicks” up each
side with a pair of scissors extending about ¼ way across the
packet as in the diagram on the left.
Put 3cm of vinegar in a test tube, drop in the packet of sodium
hydrogen carbonate and assemble the gas-tube as in the diagram
with Experiment 9.5. The ethanoic acid in the vinegar will slowly
mix with the sodium hydrogen carbonate and produce plenty of
carbon dioxide gas. Bubble this into the lime water. Does the lime
water go milky?
Experiment 9.8
To show that carbon dioxide will extinguish a flame
• sodium hydrogen
carbonate (sodium
bicarbonate)
• vinegar
• drinking glass
• matches
Carefully hold a lighted match inside an empty drinking glass.
The match continues to burn until you have to take. Be careful you
do not burn your fingers.
Put about 1cm of vinegar into the glass and add half a teaspoonful
of sodium hydrogen carbonate (sodium bicarbonate). Gently swirl
the glass as the chemicals react together until the fizzing stops.
The glass is now full of carbon dioxide gas.
Now hold a lighted match into the glass (not in the solution in the
bottom). The flame will immediately go out, because the carbon
dioxide cannot support burning. For burning to occur oxygen gas
is needed (see Experiment 9.17).
This property of carbon dioxide to extinguish flames is put to
good use in some fire extinguishers.
Chapter 9 - The Chemistry of some gases
9b - Ammonia
Chapter 9 - The chemistry of some gases
9a - Carbon dioxide
Funnel
Beaker
Test tube
with cork
rubber &
glass tubing
Spirit
burner









































