Pediflow
®
Manual
EN
medin Medical Innovations GmbH
Adam-Geisler-Str. 1
D-82140 Olching
+49 8142-44846-0
www.medingmbh.com
OP_Pediflow
®
8
(7) Regularly check the CPAP and ventilation settings and the fixation.
Important note: Regular inspection of the fixation
Regularly check the positioning and fixation of the Pediflow
®
and the accessories since pressure points may
otherwise develop.
Check the masks regularly for collected secretions since there otherwise may be an obstruction.
4.
Ventilation devices which can be connected to the Pediflow
®
The Pediflow
®
can be connected to any ventilation device which meets these conditions:
The ventilation device must offer a CPAP mode or a non-invasive mode. This mode must:
o
Control the flow and pressure such that a constant pressure is achieved in the tubing system
o
Be able to work without an external flow sensor
o
Measure the pressure inside the device or at an additional T piece in the tubing system
o
Tolerate slight leakages in the tubing system
The ventilation device must be of size F15 or M22 for the inspiration and expiration connections, then it can
be connected with the medin
®
tubing sets, or tubing sets for the ventilation device (inspiration and expiration)
must be present which have an M10 connection on the side of the Pediflow
®
.
Warning: Accessories
The Pediflow
®
only functions in combination with the corresponding mask and headgear, otherwise proper function
cannot be guaranteed.
The mask can only be combined with the nCPAP interface Pediflow
®
.
5.
Storage and disposal
The Pediflow
®
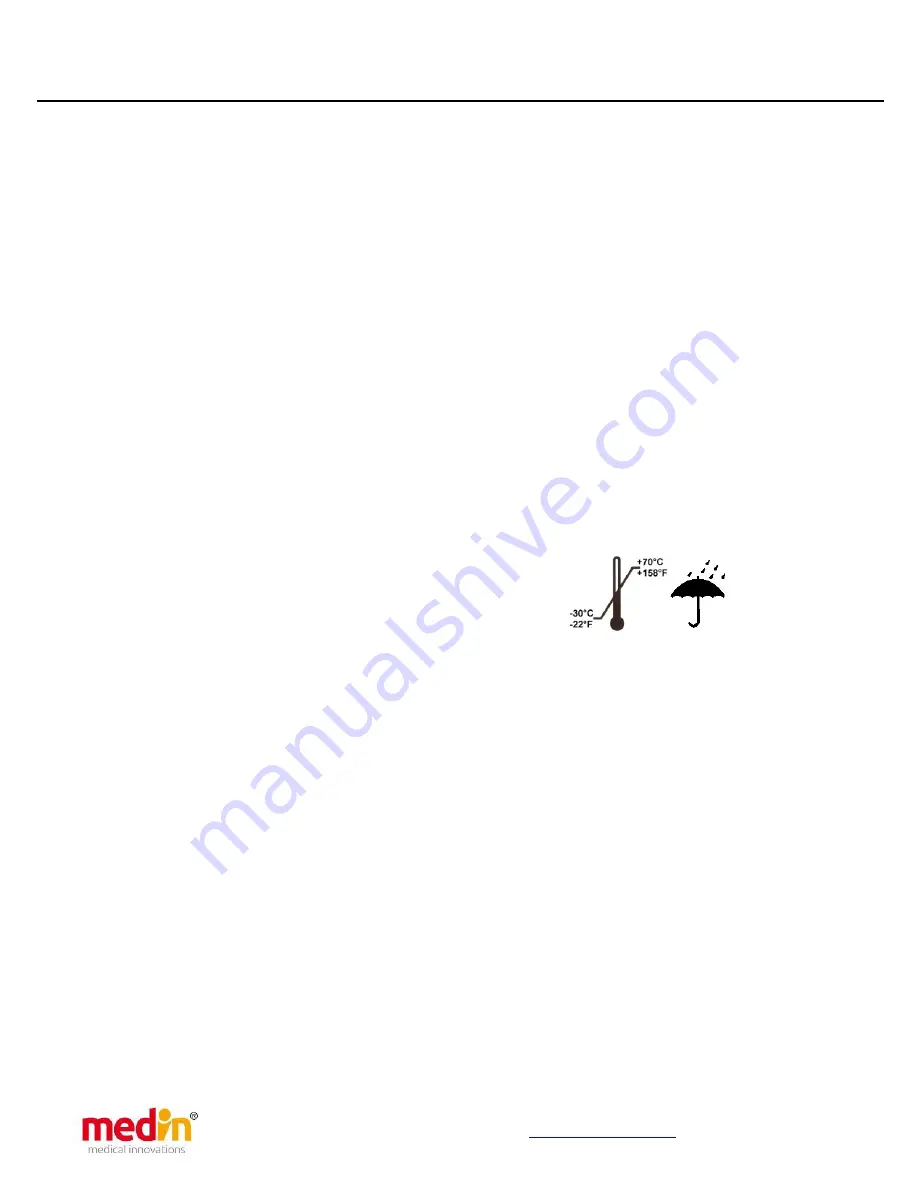
and its accessories must be stored in a
clean, dry area. Temperatures between -30°C and 70°C
have no negative effect on the Pediflow
®
or the
accessories.
The Pediflow
®
and its accessories can be discarded with
normal household waste.












































