6000-300-000 5–5
5 – Performance and Limitations
6000-300-000 5-5
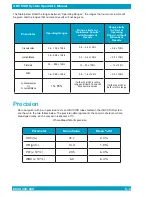
Parameter
Correlation
Coefficient
Slope
Intercept
QBC
Mean
Cell
Counter
Mean
Range of
Values
Number of
Samples
Microhematocrit (%)
0.986
1.023
–0.650
36.5
36.3
15.7 – 61.9
646
Limitations
Quality medical care requires that laboratory values be correlated with each patient’s symptoms and
signs by a trained practitioner.
Values above and below these validated ranges are not displayed or displayed with clear warning if
certain conditions are met. They should be confirmed by an alternate method.
Operating Ranges lists the validated upper and lower limits of the operating range. Values above and
below these validated ranges should be confirmed by an alternate method.
The QBC STAR reagent tube has been formulated to provide optimum packing and layering of normal
cells. In a small number of patients, however, the system cannot read certain parameters and will not
report a value. User errors in processing or use of outdated or inappropriately stored tubes can also
result in non-reported results. Practitioners must not assume that unreported values are normal; further
testing with an alternative method is essential.
Automated granulocyte and lymphocyte/monocyte differential counts cannot replace the conventional
manual differential. Due to the grouping by density of the cell populations by the QBC test method, the
system cannot discriminate between normal and abnormal cell types in disease states characterized by
the presence of abnormal white cell types or nucleated red blood cells. If abnormal cell populations are
suspected, verification of QBC test results or testing and diagnosis by alternative methods is essential.
The combined lymphocyte/monocyte count should not be used to test for lymphocytopenia in evaluat-
ing patients with known or possible immunodeficiencies. Further evaluation of lymphocyte/monocyte
counts in relevant situations must include a manual differential and lymphocyte subset analysis.
The presence of abnormally sized platelets may lead to discrepancies between the QBC test method
platelet count, which is based on platelet mass, and results obtained with an impedance counter,
which are based on measurement of particle number
12
.
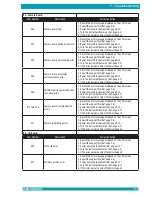
Expected Values
The following table provides normal ranges reported in the literature.
11, 13
Offices or laboratories may
choose to develop normal hematology ranges based on the characteristics of their patient population.
Parameter
Range
Hematocrit Males (%)
42.0 – 50.0
Hematocrit Females (%)
36.0 – 45.0
Hemoglobin Males (g/dL)
14.0 – 18.0
Hemoglobin Females (g/dL)
12.0 – 16.0
MCHC
(g/dL)
31.7–
36.0
Platelet Count (x10
9
/L)
140
–
440
WBC (x10
9
/L)
4.3
–
10.0
Granulocyte Count (x10
9
/L)
1.8 – 7.2
Lymphocyte/Monocyte Count (x10
9
/L)
1.7 – 4.9
Содержание QBC STAR
Страница 14: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 28: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 40: ...QBC STAR System Operator s Manual 6000 300 000 4 12 THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 50: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 72: ...6000 300 000 A 1 8 Appendices 6000 300 000 8 1...
Страница 74: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 76: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 78: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 80: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 86: ...THIS PAGE INTENTIONALLY LEFT BLANK...
Страница 89: ...THIS PAGE INTENTIONALLY LEFT BLANK...