Copyright © 2013, Condor Instruments Ltda.
63
Av. Brigadeiro Luis Antonio, 551, cj 124 - Cep:01318-000
Report
Here it is possi
ble to include wearer information’s and doctor conclusions. All
those information will be included in the PDF report file.
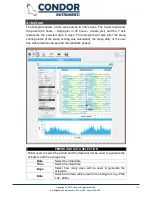
REPORT
Page used to insert some relevant information about the subject and the data
sampled.
Subject ID
Relevant identification about the subject (e.g.: name).
Date of Birth
Date of birth of the subject.
Age
Shows the subject age (read only).
Gender
Field used to select the gender of the subject.
Body Part
This field is used to select the Body Part where the
actimeter was used to collect the data. If the "none"
option was selected, this field will not be shown in the
report.
Recording Period
Field used to show the recorded period (read only).
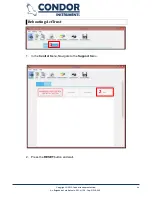
Report Settings
Open the Report Settings page.
INDICATIONS
FOR USE
This field is used to insert some relevant information
about the actimeter use for a specific subject.
SUMMARY
STATISTICS
Here you can see the summary statistics (the same
Summary Statistics that is shown in the Statistics page).
INTERPRETATION
Field used to insert some relevant interpretation of the
data sampled.