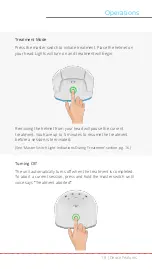
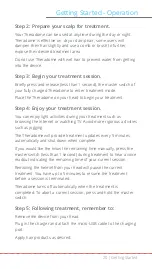
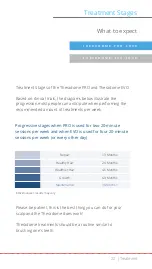
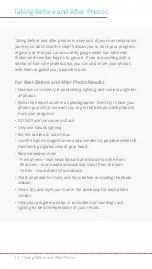
Indication for Use
The Theradome PRO and Theradome EVO devices are over-
the-counter (OTC) therapeutic devices intended to treat
Androgenetic Alopecia and promote hair growth in males who
have Hamilton-Norwood Classifications of IIa to V patterns of
hair loss and to treat Androgenetic Alopecia and promote hair
growth in females who have Ludwig-Savin Scale I-1 to I-4, II-1,
II-2; both with Fitzpatrick Skin Types I to IV.
Contraindications
Theradome devices should not be used in the case of current or
past scalp cancer. The coherent-light stimulates activity in both
healthy and unhealthy cells.
If you are undergoing treatment for cancer, we recommend you
consult your physician for any medical advice. Theradome has
not been tested on people with scalp cancer.
We do not recommend using the Theradome while pregnant or
breastfeeding. Use Theradome after you finish breastfeeding.
Theradome devices shall not be used by people with an
Ommaya reservoir under their scalp without the approval of a
specialist.
Discontinue use AT ONCE should the device appear to be
malfunctioning.
Indication for Use
8 | Intended Use