SAIT‐IFU‐GEMS‐H Rev.1.1 (2019‐11)
Page 7 of 79
2.4. Intended user profile
Occupation
•
Physical therapists
Education
•
Earned a degree in physical therapy and obtained a current physical therapist license.
Experience /
Abilities
•
Familiar with the application of the tablet PC.
•
Be able to set up the Bluetooth communication between the device and the tablet PC.
•
Complete a training program provided by Samsung on how to use the device.
2.5. Operating principle
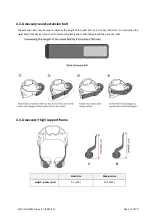
GEMS‐H is a wearable powered exoskeleton that actively assists individuals to walk. The GEMS‐H consists of snap‐
together components weighing 4.7 lbs (2.1 kg) in total. The waist part houses two actuator modules, a Bluetooth
module, and a control pack. Each actuator module houses a motor as well as an embedded angular position sensor
and controller. In the control pack, there is a central processor, an IMU sensor, a rechargeable battery pack and a
power switch.
The GEMS‐H has the ability to assist wearers’ walking. The GEMS‐H has only one Walk Assist mode, which is operated
based on the DOC (Delayed Output Control) algorithm. In the DOC algorithm, an on‐board microprocessor receives
signals from integrated sensors which provide information on the patient’s joint angles. The sensors in the device
detect the hip joint angle and associated angular velocity from the patient’s gait, based on which the central
processor determines the optimal assistive torque output. The actuator module then transmits the assistive force to
the patient’s hip joint to reduce the physical effort in walking.
FCC information:
‐ Frequency range & Number of channels: 2 402
㎒
~ 2 480
㎒
_Bluetooth (79 ch)
‐ Modulation: GFSK, π/4DQPSK, 8DPSK
‐ Max. Tune‐up power: 0.5 dBm
2.6. Contraindications
Severe concurrent medical diseases: infections, circulatory, heart or lung, pressure sores
Lower extremity fracture
Heterotopic ossification or severe osteoporosis
Psychiatric or cognitive situations that may interfere with proper operation of the device
Pregnancy
For patients with a weight of 100 kg (220 lb) or more, or BMI ≥ 35 kg/ m
2
, use of the GEMS‐H is not prohibited, but the
GEMS‐H may only be used for those patients after careful consultation with medical professionals.
Summary of Contents for GEMS-H
Page 1: ...GEMS H Instructions for Use ...
Page 19: ...SAIT IFU GEMS H Rev 1 1 2019 11 Page 19 of 79 4 6 Marking plate ...
Page 52: ...SAIT IFU GEMS H Rev 1 1 2019 11 Page 52 of 79 6 7 3 3 Example of parameter control ...
Page 54: ...SAIT IFU GEMS H Rev 1 1 2019 11 Page 54 of 79 Error message examples ...
Page 64: ...SAIT IFU GEMS H Rev 1 1 2019 11 Page 64 of 79 ...
Page 67: ...SAIT IFU GEMS H Rev 1 1 2019 11 Page 67 of 79 ...