Chapter 2 Compatible Reprocessing Methods and Chemical Agents
11
EVIS EXERA GIF/CF/PCF TYPE 160 Series REPROCESSING MANUAL
• Before sterilization, the instrument must be thoroughly
cleaned and dried. Residual moisture inhibits sterilization.
• The results of sterilization depend on various factors such as
how the sterilized instrument was packed or the positioning,
method of placing and loading of the instrument in the
sterilization device. Please verify the sterilization effects by
using biological or chemical indicators. Also follow the
guidelines for sterilization issued by medical administrative
authorities, public organizations or the infection management
sections at each medical facility, as well as the instruction
manual of the sterilization device.
• All instruments must be properly aerated following ETO gas
sterilization to remove toxic ethylene oxide residuals.
• Exceeding the recommended parameters may cause
equipment damage.
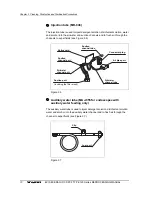
• When reprocessing EVIS videoscope models, remove the
water-resistant cap before ETO gas sterilization (see Figures
2.1 and 2.2).
• Repeated ETO gas sterilization procedures will gradually
deteriorate the instrument. Do not perform ETO gas
sterilization to the instrument unnecessarily.
ETO gas exposure parameters
Process
Parameters
ETO gas sterilization
Temperature
57
C (135
F)
Pressure
0.1 – 0.17 MPa
(1 – 1.7 kgf/cm
2
)
(16 – 24 psig)
Humidity
55%
Exposure time
1.75 hours
ETO gas
concentration
0.6 – 0.7 mg/cm
3
(600 – 700 mg/l)
Aeration
Minimum aeration
parameters
12 hours in an aeration chamber
at 50 – 57
C (122 – 135
F) or
7 days at room temperature
Table 2.2