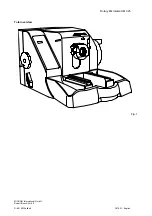
Rotary Microtome HM 325
MICROM International GmbH
Robert-Bosch-Str. 49
D- 69190 Walldorf
387821 - English
EC Certificate of Conformity
Name and address of MICROM International GmbH
the manufacturer:
Robert-Bosch-Straße 49
D-69190
Walldorf
Product designation: Rotary Microtome
Type reference:
HM 325 -2
Notification to Competent Authorities:
These medical devices have been registered with the German authority as
“Microtomes” under the EDMA-classification code: 23-06-02
The designated product complies with the laid down regulation:
DIRECTIVE 98/79/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
of 27 October 1998
on in vitro diagnostic medical devices
The designated product complies with the EC regulations by strictly observing the
following norms:
DIN EN ISO 14971:2001-03
Medical devices - Application of risk management to medical devices (ISO 14971:2000).
DIN EN ISO 9001:2000
Quality management systems - Requirements (ISO 9001:2000)