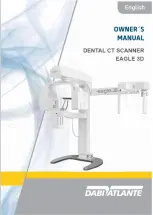
6
0482
CE Mark: The
Product system
conforms to essential
requirements of the
Medical Device
Directive 93/42/EEC.
Non-hook
Date of manufacture.
Specifies serial
number of the
Product
Manufacturer
LOT Number
Storage Temperature
It indicates that the
equipment should
be sent to the
special agencies
according to local
regulation for
separate collection
after its useful life.
Type BF applied part
Ingress
Protection.
SECTION 3: USING THE PRODUCT
This section presents information on unpacking and setting up the
product.
3.1 UNPACKING AND INSPECTING
Every attempt is made to ensure your accurate and complete order.
However, to be sure that your correct order, verifying the contents of the
box against your packing slip.
The product is designed for simplicity of operation and set-up and
requires minimal assembly. The following items are included in your
box: