35
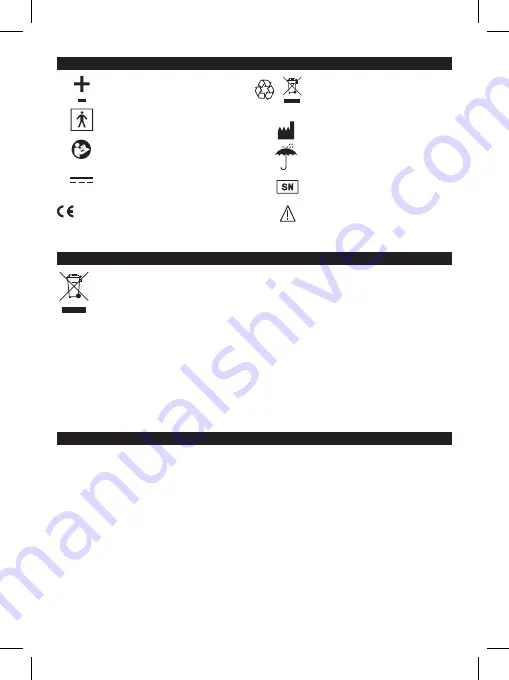
11 - SYMBOLS
0051
Positive batteries polarity
Negative batteries polarity
Type BF applied parts
WARNING! Check the
instructions for use manual
Direct current
CE Marking medical ref.
Dir 93/42 EEC Directive and
subsequent updates
Battery disposal: Used up
batteries must be disposed
of in appropriate containers.
Manufacturer
Keep dry
Serial number
of the device
Warning
12 - DISPOSAL OF DEVICE
13 - ELECTROMAGNETIC COMPATIBILITY
In conformity with Directive 2012/19/EC, the symbol shown on the device
to be disposed of indicates that it is considered as waste and is therefore
subject to “sorted waste collection”. The user must therefore take (or have
taken) the above waste to a pre-sorted waste collection centre set up by
the local authorities, or else give it back to the dealer when purchasing a new
appliance of the same type. Pre-sorted waste collection and the subsequent
treatment, recovery and disposal operations favor the production of appliances
made of recycled materials and limit the negative effects of any incorrect waste
management on the environment and public health. The unlawful disposal of
the product by the user could result in administrative fines as provided by the
laws transposing Directive 2012/19/EC of the European member state or of the
country in which the product is disposed of.
This device has been designed to satisfy requirements currently required for
electromagnetic compatibility (EN 60 601-1-2:2007). Electrical medical devices
require special care during installation and use with respect to EMC requirements.
It is therefore required that they be installed and/or used according to the
manufacturer’s specification. Potential risk of electromagnetic interference
with other devices. Radio and mobile telecommunications devices or portable
RF (mobile phones or wireless connections) may interfere with the operation
of electrical medical devices. For further information visit www.flaemnuova.
it website. The Device may be subject to electromagnetic interference if other
devices are used for specific diagnosis or treatments. Flaem reserves the right
to make technical and functional modifications to the product without notice.
Summary of Contents for fifty
Page 38: ...37 NOTE...
Page 39: ...38 NOTE...


























