Interferences
Most cations and anions do not interfere with the response of
the fluoride electrode to fluoride. Anions commonly associated
with fluoride, such as Cl
-
, Br
-
, I
-
, S0
4
2-
, HC0
3
-
, P0
4
3-
, and acetate,
do not interfere with electrode operation. The OH
-
ion is an
electrode interference. See
pH Effects.
Some anions, such as
C0
3
=
or P0
4
-3
, make the sample more basic, increasing the
OH
-
interference, but are not direct electrode interferences.
pH Effects
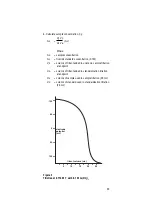
In acid solutions with a pH below 5, hydrogen complexes a
portion of fluoride in solution, forming the undissociated acid HF
and the ion HF
2
-
.
Figure 4
shows the proportion of free fluoride
ion in acid solutions. Hydroxide ion interferes with the electrode
response to fluoride when the level of hydroxide is greater than
one-tenth the level of fluoride ion present. For example, at pH 7,
when the hydroxide concentration is 10
-7
M or less, there is no
hydroxide interference with fluoride measurements. At pH 10,
where the hydroxide concentration is 10
- 4
M, there is no error at
10
-2
M fluoride, about a 10% error at 10
-4
M fluoride and
considerable error at 10
-5
M fluoride. See
Figure 5.
Addition of
TISAB II or III to fluoride standards and samples will buffer the
pH between 5.0 and 5.5 to avoid hydroxide interferences or the
formation of hydrogen complexes of fluoride. TISAB IV
adjusts the pH to about 8.5, and should not be used for very
low-level measurements.
35
Содержание 96-09
Страница 7: ...4 ...