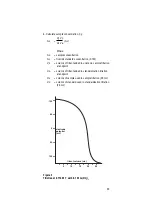
Standard
The quality of results depends greatly upon the quality of the
standards-
ALWAYS prepare fresh standards when problems
arise
- it could save hours of frustrating troubleshooting! Error
may result from contamination of prepared standards, accuracy
of dilution, quality of distilled water, or a mathematical error in
calculating the concentrations.
The best method for preparation of standards is by serial
dilution. This means that an initial standard is diluted, using
volumetric glassware, to prepare a second standard solution.
The second standard solution is similarly diluted to prepare a
third standard, and so on, until the desired range of standards
has been prepared.
Sample
If the electrodes work properly in standards but not in sample,
look for possible interferences, complexing agents, or
substances, which could affect response or physically damage
the sensing electrode or the reference electrode. If possible,
determine the composition of the samples and check for
problems. See
Sample Requirements, Interferences,
and
pH Requirements.
Technique
Check the method of analysis for compatibility with your
sample. Direct measurement may not always be the method of
choice. If a large amount of complexing agents are present,
known addition may be best. If the sample is viscous, analate
addition may solve the problem. If working at low-level, be sure
to follow the
Low-Level Measurement Technique.
Also, be sure that the expected concentration of the ion of
interest is within the electrode’s limits of detection.
It problems persist, review operational procedures and
instruction manuals to make sure that proper technique has
been to followed. Reread
Measuring Hints
and
Analytical Procedures.
31
Содержание 96-09
Страница 7: ...4 ...