-31-
-32-
Maintenance
1) We do not authorize any institution or individual to maintain
and/or repair the product. If you suspect that the products may be
in question, please contact us immediately.
2) The user must not attempt any repairs to the device or any of its
accessories. Please contact us for any repair needs.
3) Opening of the equipment by unauthorized agencies is not
allowed and will terminate any claim to warranty. Warning: No
modification of this equipment is permitted.
Storage
1) Always store your devices in the Charging Case when not in
use.
2) Do not store or expose the devices and/or case to extreme
hot/cold temperatures, moist/humid environments, or shock/vibra-
tion.
3) Keep the devices and case in following environmental ranges:
-10
℃~
55
℃
, 10%-90%RH, 700-1060 hPa
XII
.
MAINTENANCE, STORAGE, AND DISPOSAL
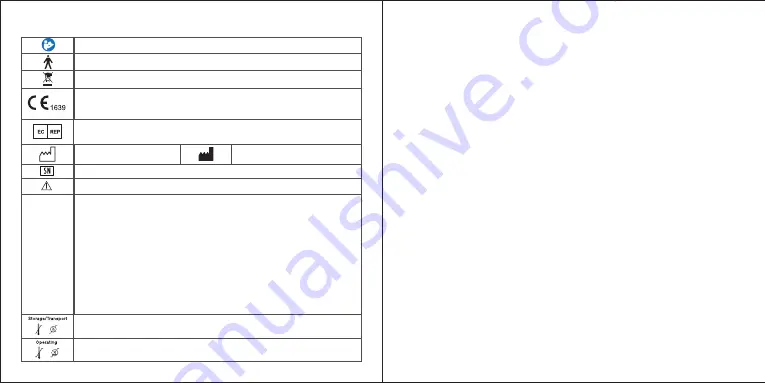
XI. NORMALIZED SYMBOLS
Test Condition:
Access to hazardous parts :The test wire of 10 mm Ø shall not
penetrate and adequate clearance shall be kept.
No dust entered the sample after testing the function is normal.
IPX3 Test Condition:
Water flow rate:0.07L/min±5% per multiplied by Number of holes
10L/min±5%
Duration of test: 10min 1 min/m2 at least 5 min
Test Condition:14.2.3a) 14.2.3b)
There is no water enter the sample inside. The function is normal
after the test.
IP53
Follow operating instructions
B type applied part
Disposal in accordance with Directive 2002/96/EC (WEEE)
Date of manufacture
Serial number
Warning/ Danger: Improper use might cause serious injuries.
Permissible storage and transport temperature and humidity
Permissible operating temperature and humidity
Complies with the European Medical Device Directive (93/42/EEC and
amended Directive 2007/47/EC. Notified Body is SGS Belgium.
Manufacturer information
Shanghai International Holding Corp. GmbH(Europe)
Address:Eiffestrasse 80,20537 Humburg,Germany
Notes:This hearing aid is produced under the strict quality system
of Huizhou JINGHAO Medical Technology Co.,Ltd.
The device fulfils the provisions of the EC directive 93/42/EEC
(Medical Device Directive) and the Harmonize Standard as IEC
60601-1, IEC 60601-2-66, IEC 60118-7, IEC 60118-13, EN ISO
10993-1/-5/-10 and EN ISO 14971.
















